Synthetic method of high-purity azilsartan medoxomil impurity
A technology of azilsartan medoxomil and a synthetic method, applied in the synthesis field of high-purity azilsartan medoxomil impurity, can solve the problems such as no impurity I purification method reported in the literature, no specific report impurity purification method, etc., and achieves high purity, Simple synthesis and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
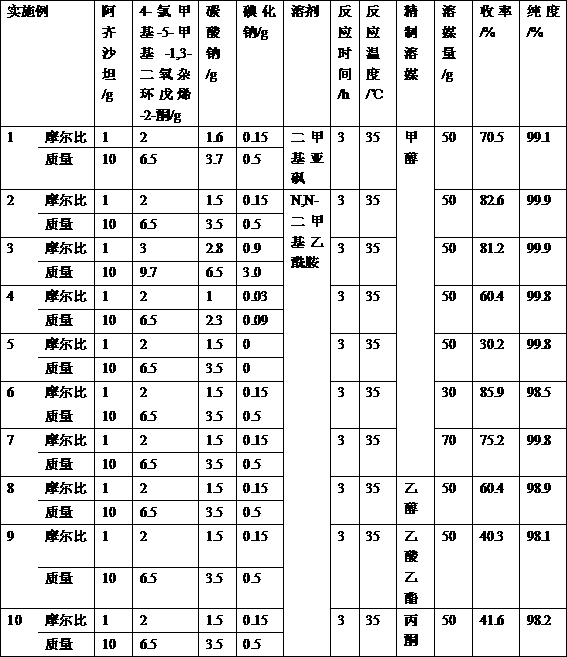
[0023] Embodiment 1: add 10g Azilsartan, 6.5g 4-chloromethyl-5-methyl-1,3-dioxol-2-one, 3.7g potassium carbonate, 0.5g iodine in reaction flask Sodium chloride and 45g dimethyl sulfoxide were heated to 35°C for 3h.
[0024] After the reaction is completed, filter, add 50g of dichloromethane and 50g of purified water to the filtrate, after separation, extract the water layer once with 20g of dichloromethane, combine the dichloromethane layers, wash with brine once, concentrate under reduced pressure, add 50g of methanol was stirred at 30°C for 5h, and the crude impurity was obtained by filtration.
[0025] Add 30 g of methanol to the crude product, stir to raise the temperature, filter, cool down to crystallize, filter, and dry to obtain 10.5 g of refined azilsartan medoxomil impurity, with a total yield of 70.5% and a liquid phase purity of 99.1%.
Embodiment 2
[0026] Example 2: 10g Azilsartan, 6.5g 4-chloromethyl-5-methyl-1,3-dioxol-2-one, 3.5g sodium carbonate, 0.5g iodine were added to the reaction flask Sodium chloride and 45g N,N-dimethylacetamide were heated to 35°C for 3h.
[0027] After the reaction is completed, filter, add 50g of dichloromethane and 50g of purified water to the filtrate, after separation, extract the water layer once with 20g of dichloromethane, combine the dichloromethane layers, wash with brine once, concentrate under reduced pressure, add 50g of methanol was stirred at 30°C for 5h, and the crude impurity was obtained by filtration.
[0028] Add 30 g of methanol to the crude product, stir to raise the temperature, filter, cool down to crystallize, filter, and dry to obtain 12.3 g of fine azilsartan medoxomil impurity, with a total yield of 82.6% and a liquid phase purity of 99.9%.
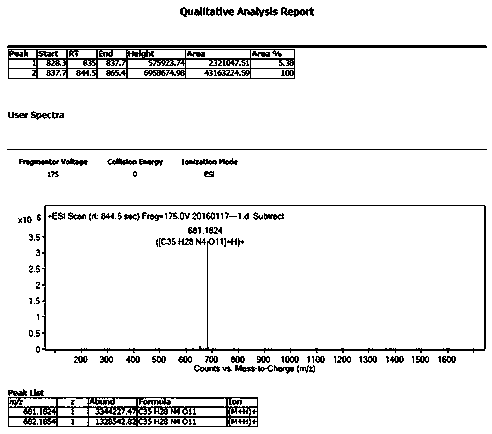
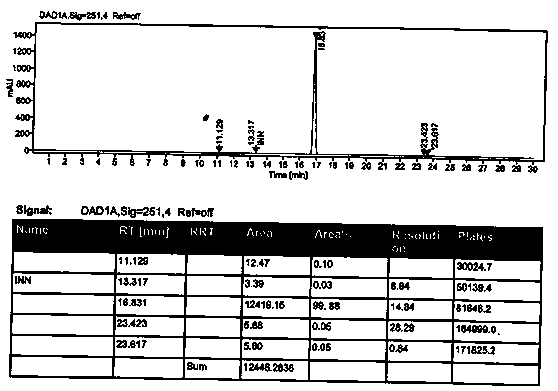
[0029] The product is identified by mass spectrometry: figure 1 It is the mass spectrum of azilsartan medoxomil impurity I...
Embodiment 3
[0039] Example 3: 10g Azilsartan, 9.7g 4-chloromethyl-5-methyl-1,3-dioxol-2-one, 6.5g sodium carbonate, 3.0g iodine were added to the reaction flask Sodium chloride and 45g N,N-dimethylacetamide were heated to 35°C for 3h.
[0040] After the reaction is completed, filter, add 50g of dichloromethane and 50g of purified water to the filtrate, after separation, extract the water layer once with 20g of dichloromethane, combine the dichloromethane layers, wash with brine once, concentrate under reduced pressure, add 50g of methanol was stirred at 30°C for 5h, and the crude impurity was obtained by filtration.
[0041] Add 30 g of methanol to the crude product, stir to raise the temperature, filter, cool down to crystallize, filter, and dry to obtain 12.1 g of fine azilsartan medoxomil impurity, with a total yield of 81.2% and a liquid phase purity of 99.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com