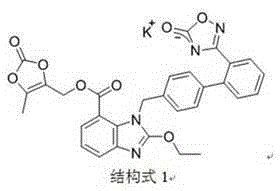
New preparation method of azilsartan kamedoxomil
A technology for azilsartan medoxomil and potassium salt, applied in the field of preparing azilsartan medoxomil potassium salt, can solve the problems of poor solubility of azilsartan medoxomil, unfavorable for industrialized production, easy to exceed the standard of residue on ignition, etc. The effect of poor solubility, simple operation and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
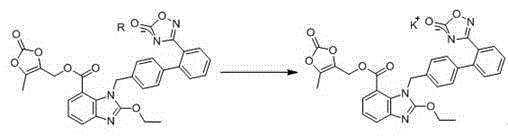
[0030] Preparation of the complex: Add 5 g of azilsartan medoxomil and 70 ml of ethyl acetate into a dry reaction bottle. Stir to lower the temperature, and add 1 g of triethylamine dropwise at 10-15°C. Stir to dissolve, and obtain the ethyl acetate solution of azilsartan medoxomil triethylamine salt. This intermediate was directly carried on to the next step without treatment.
[0031] Add dropwise 20ml of ethyl acetate solution of 1.8g potassium isooctanoate to the ethyl acetate clear solution of azilsartan medoxomil triethylamine salt at 10~15°C. After the dropwise addition was completed, stir for 1 h, crystallize, and filter to obtain 5.0 g of azilsartan medoxomil potassium salt with a yield of 94.1% and a purity of 99.6%. mp: 209.4~210.8°C, (literature data: 209~213°C).
[0032] 1 HNMR (DMSO-d6) б1.37-1.41 (3H, t), 2.16 (3H, s), 4.57-4.63 (2H, q), 5.12 (2H, s), 5.55 (2H, s), 6.86-6.88 (2H, d), 7.19-7.46 (4H, m), 7.52-7.54 (2H, m), 7.62-7.63 (2H, m), 1.37-1.41 (3H, m)...
Embodiment 2
[0034] Preparation of the complex: Add 10 g of azilsartan medoxomil and 75 ml of acetone into a dry reaction bottle. Stir to lower the temperature, and add 1.5 g of pyridine dropwise at 15-20°C. Stir to dissolve, and obtain the acetone solution of azilsartan medoxomil pyridinium salt. This intermediate was directly carried on to the next step without treatment.
[0035] At 15~20°C, 20ml of acetone solution containing 3.4g of potassium isooctanoate was added dropwise to the clear solution of azilsartan medoxoproxil pyridinium salt in acetone, after the dropwise addition was completed, stirred for 1 hour, crystallized and filtered to obtain Azilsartan Ester potassium salt 9.96g, yield 93.1%, purity 99.4%. mp: 208.9~210.7°C.
Embodiment 3
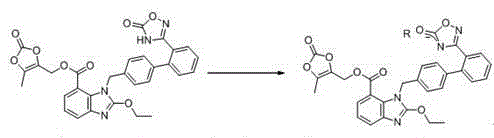
[0037] Preparation of the complex: Add 9.5 g of azilsartan medoxomil and 75 ml of acetone into the dry reaction bottle. Stir to lower the temperature, and add 1.8 g of triethylamine dropwise at 12-17°C. Stir to dissolve, and obtain the acetone solution of azilsartan medoxomil triethylamine salt. This intermediate was directly carried on to the next step without treatment.
[0038] Add dropwise 20ml of acetone solution of 3.4g potassium isooctanoate to the acetone clarified solution of azilsartan medoxomil triethylamine salt at 12~17°C. After the dropwise addition was completed, stir for 2 hours, crystallize, and filter to obtain 9.6 g of azilsartan medoxomil potassium salt, with a yield of 95.05% and a purity of 99.7%. mp: 208.4~210.5°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com