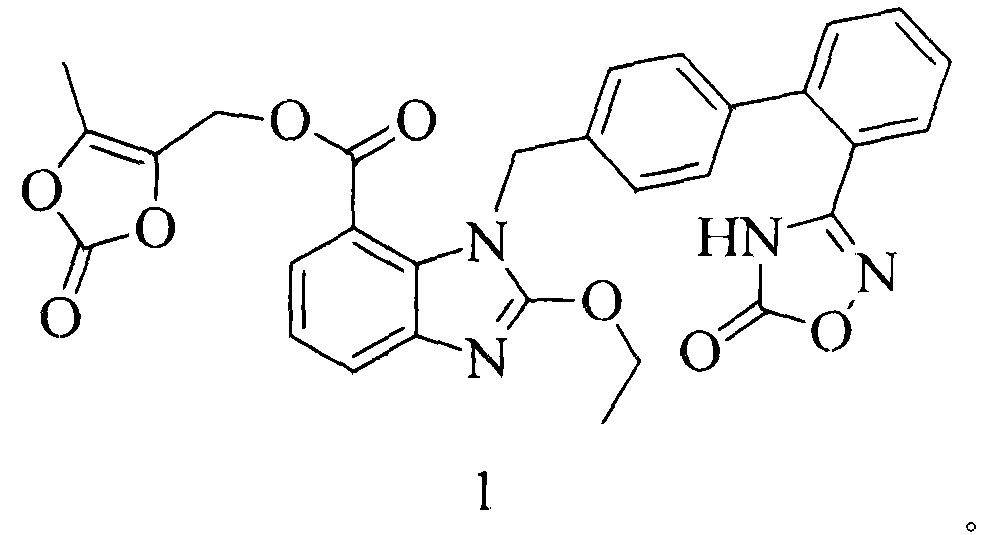
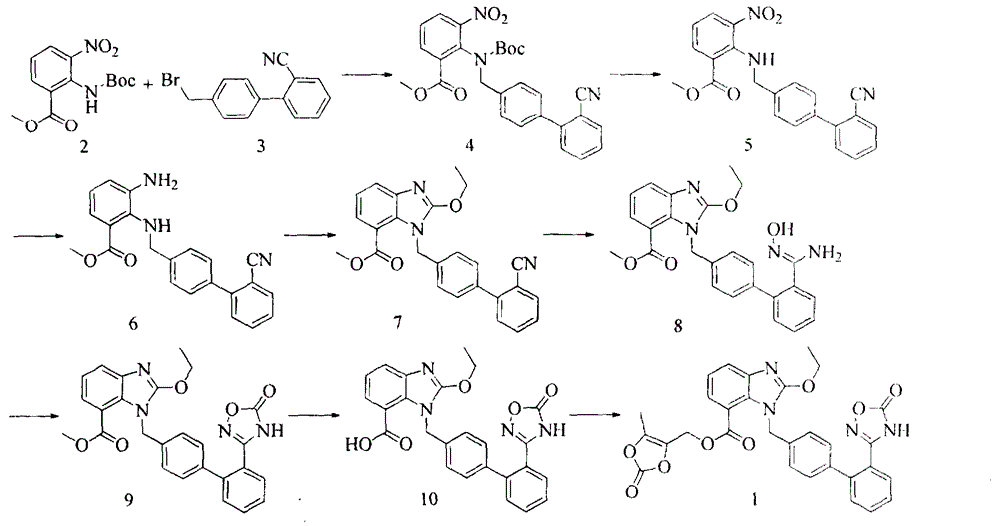
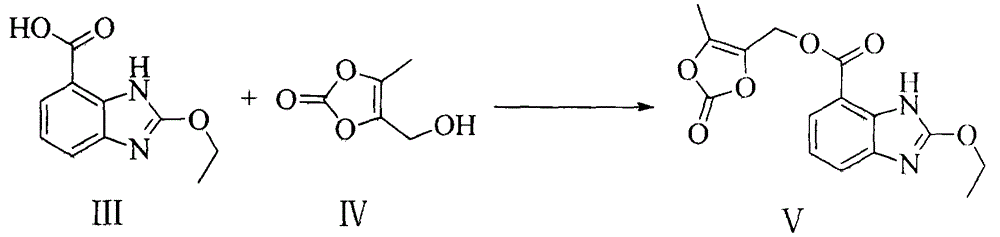
Azilsartan medoxomil preparation method
A technology of azilsartan medoxomil and compounds, applied in the field of hypertension drug synthesis, can solve the problems of long routes, increased consumption, increased production costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of embodiment one 4'-bromomethyl-2-cyanobiphenyl
[0015]
[0016] 206g2-ethoxybenzimidazole-7-carboxylic acid and 195g4-(hydroxymethyl)-5-methyl-[1,3]dioxol-2-one formula IV are dissolved in 2LN, N - In dimethylformamide, cool the mixture to 0-5°C, add 207g of potassium carbonate, 230g of p-toluenesulfonyl chloride and 35g of 4-dimethylaminopyridine in sequence, and react at 0-5°C for 1 hour after the addition is complete. Raise the temperature to 15°C and continue to stir the reaction for 12 hours. After the reaction was detected by TLC, adjust the pH to 5 with 0.5 mol / L hydrochloric acid, add 2L ethyl acetate and 2L water layer, and wash the ethyl acetate layer with 1L water Wash twice, concentrate the organic phase to obtain a solid that was refluxed with 500 mL of methanol for 4 h, cooled to room temperature and filtered, and the filter cake was further refluxed with 500 mL of methanol for 4 h, cooled to room temperature for filtration, and dried...
Embodiment 2
[0023] The preparation of embodiment two 2-ethoxybenzimidazole-7-carboxylic acid
[0024] References for the preparation of 2-ethoxybenzimidazole-7-carboxylic acid AFacile One-pot Synthesis of Benzimidazoles from 2-Nitroanilines by ReductiveCyclization (Heterocycles, 2008, 8(75): 1907-1911).
Embodiment 3
[0025] The preparation of embodiment three azilsartan medoxomil
[0026] Step 1) Preparation of Compound I
[0027]
[0028] At room temperature, add 250 g of hydroxylamine hydrochloride and 370 g of sodium bicarbonate to 5 LN, N-dimethyl sulfoxide solvent, stir to dissolve it, heat the mixture to 50 ° C, and add 100 g of 4′-bromomethyl- 2-cyanobiphenyl, after the addition, the temperature of the mixture was raised to 90-95°C to continue the reaction for 48 hours, the reaction progress was detected by TLC method, cooled to room temperature, filtered, the filtrate was poured into 10L water, continued to stir for 1 hour, filtered, filtered The cake was washed with 1L of water and dried to obtain 96g of compound I with a yield of 85.6%.
[0029] 1 H-NMR (400MHz, MEOD) δ 4.76 (s, 2H), 7.21-7.87 (m, 8H). LC-MS: m / z = 306.1 (C 14 h 13 BrN 2 O+H + ).
[0030] Step 2) Preparation of Compound II
[0031]
[0032] At room temperature, 150 g of compound I was dissolved in 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com