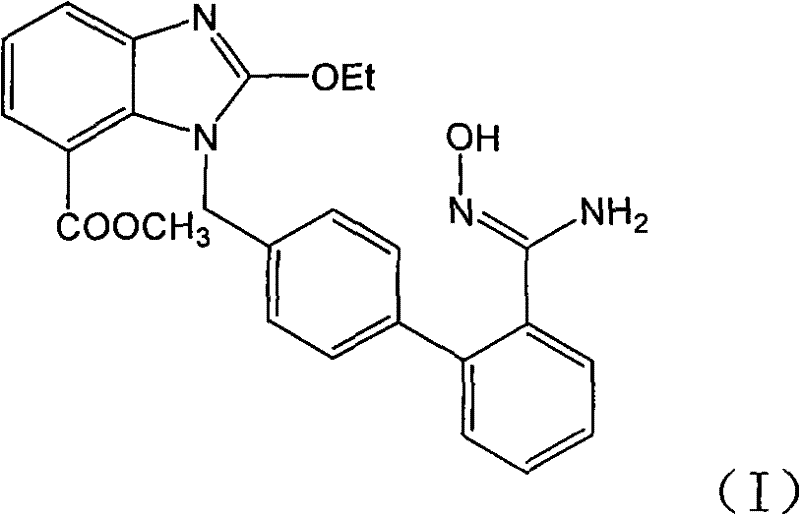
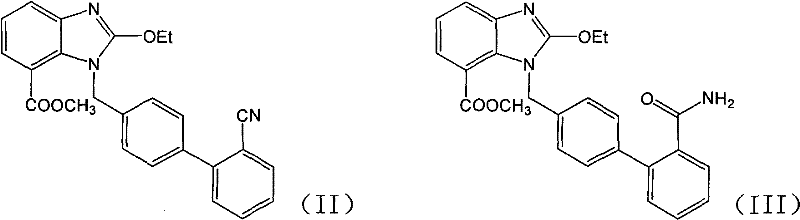
Preparation method of azilsartan intermediate
A technology of ethoxy and hydroxyaminomethimino, which is applied in the field of preparation of azilsartan intermediates, can solve the problems of impossibility and low yield, and achieve the goal of improving efficiency, increasing yield and reducing amide impurities Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-carboxylic acid methyl ester (0.4g, 0.97mmol), 50% aqueous solution of hydroxylamine (0.33g , 4.9mmol), triethylamine (0.1g, 0.97mmol) in ethanol (10ml) was refluxed for 48h, cooled and crystallized, filtered to obtain the title compound 0.2g, yield 46.3%. After 48 hours of reaction, the liquid phase data of the reaction liquid showed that amide impurity: product (area ratio of liquid phase spectrum): raw material = 37.37: 54.52: 0.42.
Embodiment 2
[0020] 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-carboxylic acid methyl ester (2g, 4.9mmol), 50% aqueous hydroxylamine solution (3.43g, 49mmol), triethylamine (0.5g, 4.9mmol) in ethanol (20ml) was refluxed for 24h, cooled and crystallized, and filtered to obtain 1.52g of the title compound with a yield of 70.4%. After 24 hours of reaction, the liquid phase data of the reaction liquid showed that amide impurity: product (area ratio of liquid phase spectrum): raw material = 5.04:79.66:0.
Embodiment 3
[0022] 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-carboxylic acid methyl ester (0.4g, 0.97mmol), 50% aqueous solution of hydroxylamine (1.0g , 14.5mmol), triethylamine (0.1g, 0.97mmol) in ethanol (10ml) was refluxed for 20h, cooled and crystallized, and filtered to obtain 0.31g of the title compound with a yield of 71.7%. After 20 h of reaction, the liquid phase data of the reaction liquid showed that amide impurity: product (area ratio of liquid phase spectrum): raw material = 7.17:78.2:0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com