Crystal form of azilsartan and preparation method thereof
A crystal form and X-ray technology, applied in the field of azilsartan's crystal form and its preparation, can solve the problems of poor purity, poor solubility, poor stability, etc., and achieve high yield, high purity and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] 2-ethoxy-1-([2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl) -Methyl benzimidazole-7-carboxylate (10g) was dissolved in methanol (727.3ml), and 62ml of 2N LiOH aqueous solution was added, then heated to reflux for 3 hours, the pH value of the solution was adjusted to 3 with 2N hydrochloric acid, and evaporated to dryness solvent. The obtained residue was distributed between water (1.2L) and chloroform (3L), then the organic layer was washed with water and dried, and the solvent was evaporated to dryness. After completely dissolving, cool to room temperature and stir to crystallize to obtain 8 g of off-white solid.
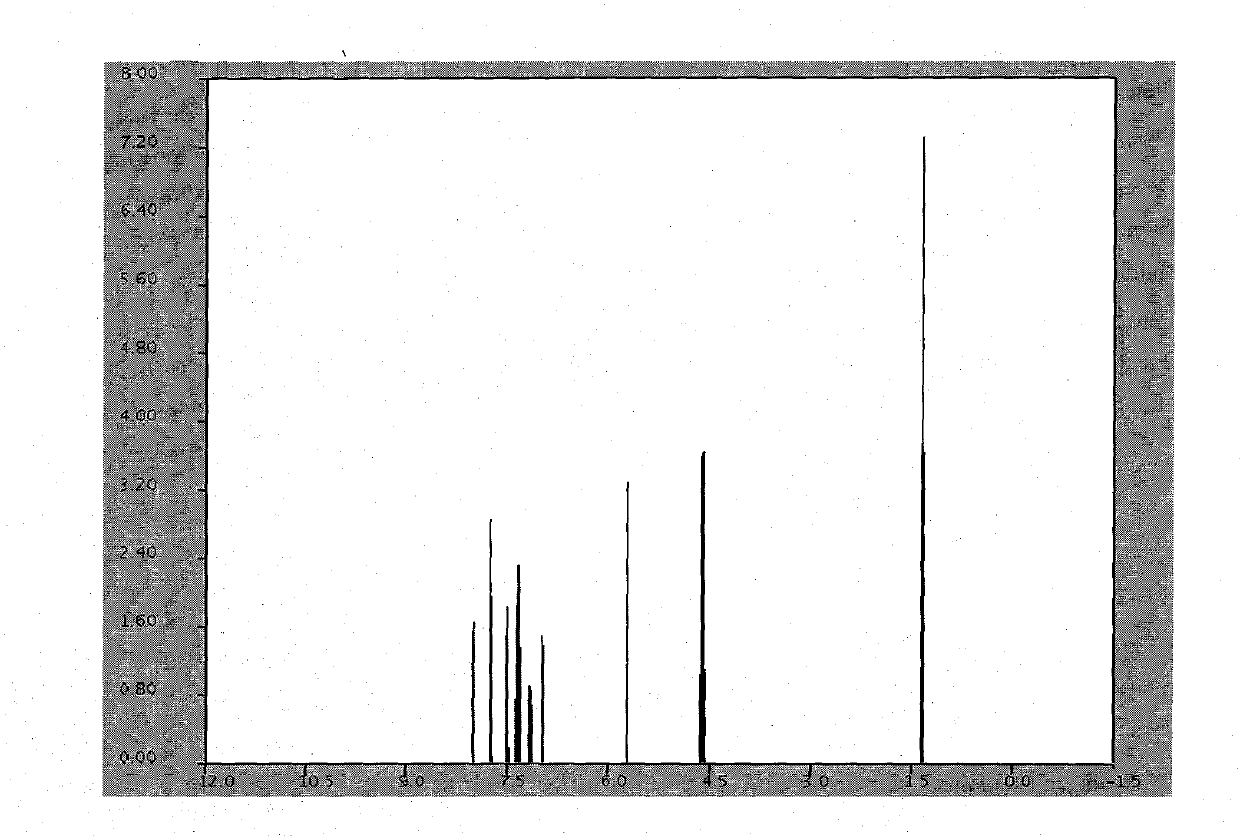
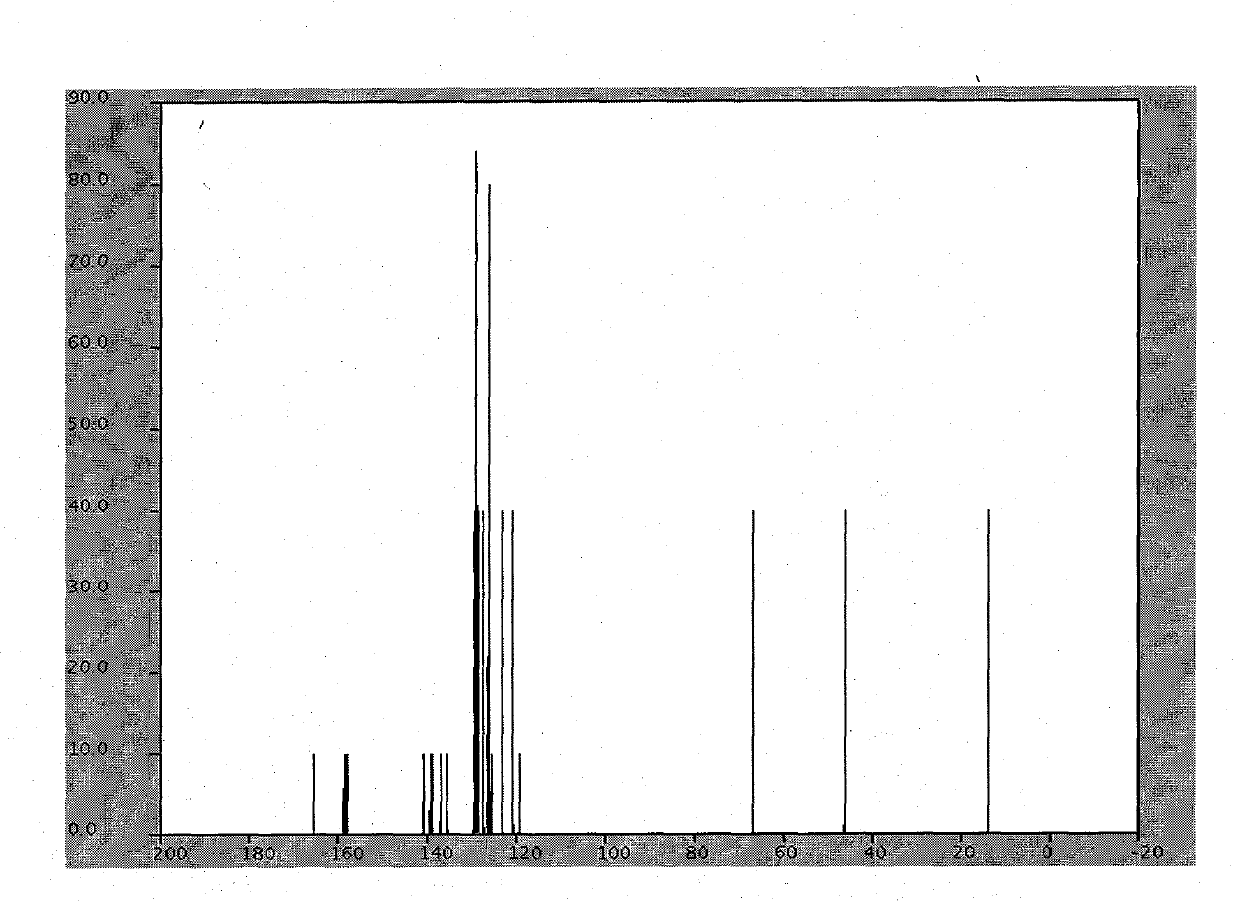
[0016] combine figure 2 , image 3 The white solid was identified as azilsartan.
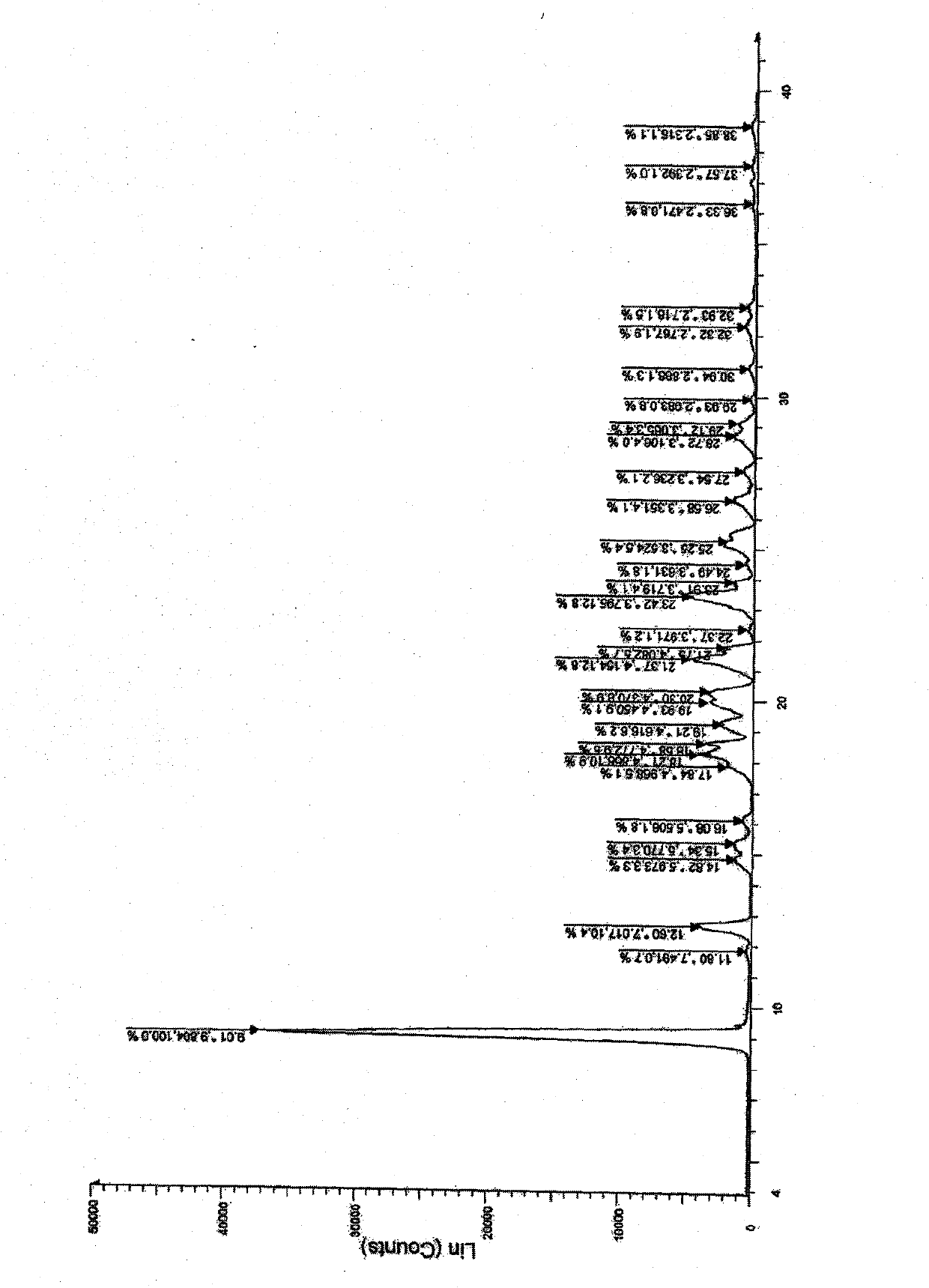
[0017] combine figure 1 The crystal form X-ray diffraction diagram of the present invention is compared with the crystal form spectrum of the prior art, and it can be found that the crystal form of the present invention is a new crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com