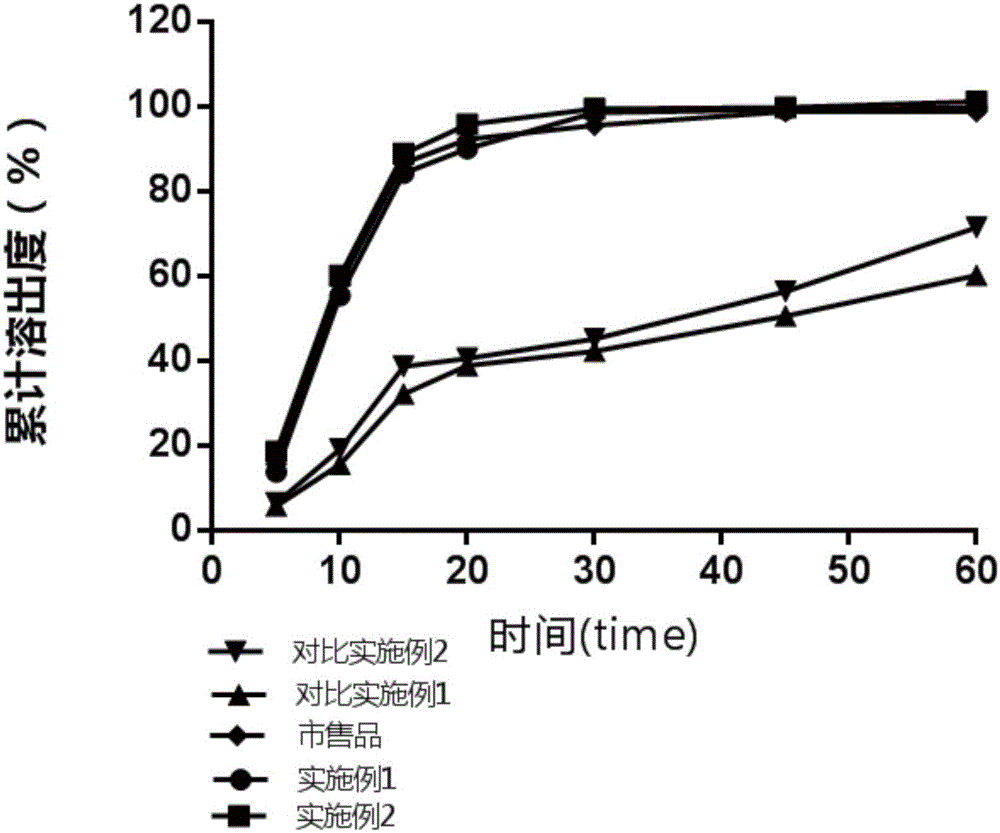
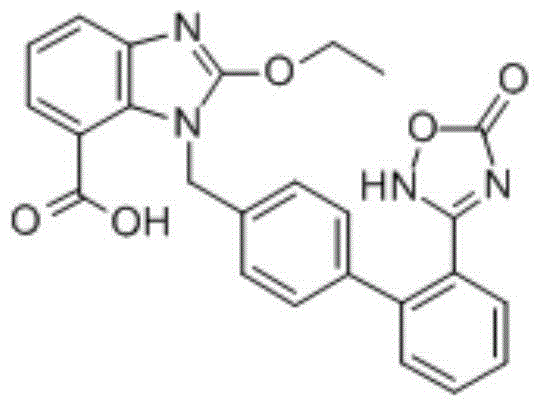
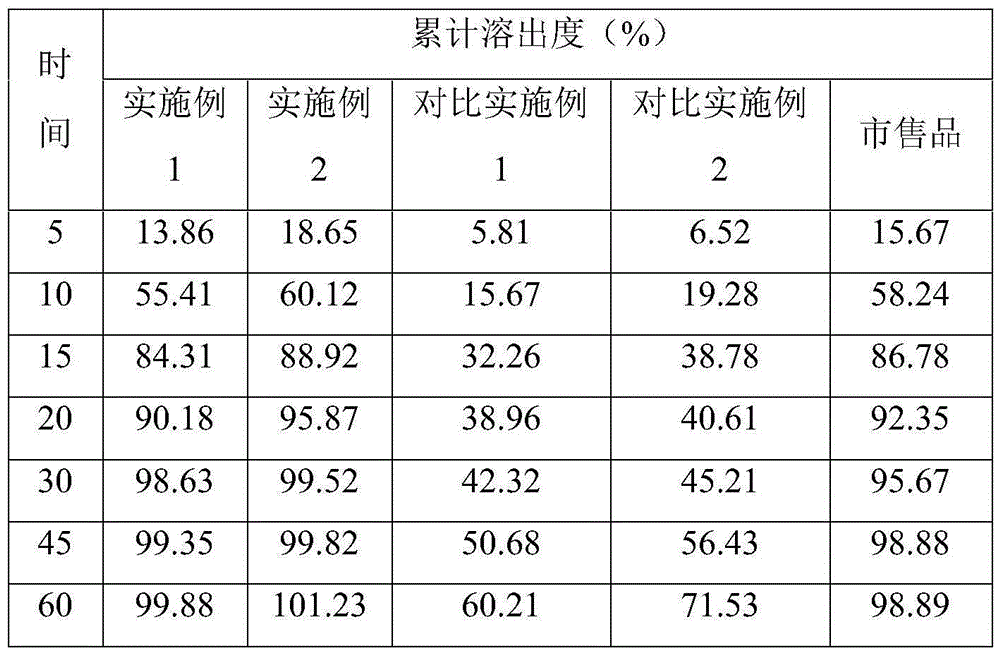
Preparation method of Azilsartan tablets
A technology of azilsartan tablet and disintegrant, which is applied in the field of medicine and achieves the effects of accelerated dissolution rate, consistent dissolution curve and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Azilsartan 20g
[0030] Lactose monohydrate 120g
[0031] Starch 30g
[0032] Hypromellose E57g
[0033] Low-substituted hypromellose 15g
[0034] Polyethylene glycol 60006g
[0035] Magnesium stearate 1.0g
[0036] Preparation Process:
[0037] (1) Azilsartan is micronized, control D90<15 microns, and the prescription amount is weighed;
[0038] (2) Lactose monohydrate is passed through a 60-mesh sieve, and the prescription amount is weighed;
[0039] (3) Pass low-substituted hypromellose and starch through a 60-mesh sieve respectively, and weigh the prescription amount;
[0040] (4) Mix (1), (2), and (3), then add polyethylene glycol 6000 in equal amounts and mix, pass through a 60-mesh sieve and mix well; after mixing the above raw and auxiliary materials, add hydroxypropyl Soft material made of methyl cellulose E5; granulated by sieving, dried and granulated, then added with magnesium stearate, mixed evenly, and compressed into tablets.
Embodiment 2
[0042] Azilsartan 20g
[0043] Lactose monohydrate 140g
[0044] Starch 30g
[0045] Sodium carboxymethyl starch 8g
[0046] Povidone K304g
[0049] Sodium Lauryl Sulfate 6g
[0050] Preparation Process:
[0051] (1) Azilsartan is micronized, control D90<15 microns, and the prescription amount is weighed;
[0052] (2) Lactose monohydrate is passed through a 60-mesh sieve, and the prescription amount is weighed;
[0053] (3) Pass hydroxymethyl starch sodium and starch through a 60-mesh sieve respectively, and weigh the prescription amount;
[0054] (4) Mix (1), (2) and (3), then add sodium lauryl sulfate in equal increments and mix, pass through a 60-mesh sieve and mix evenly; after mixing the above raw and auxiliary materials, add povidone K30 soft material; sieve and granulate, dry and granulate, then add magnesium stearate and talcum powder, mix evenly, and compress into tablets.
Embodiment 3
[0056] Azilsartan 20g
[0057] Lactose monohydrate 120g
[0058] Croscarmellose Sodium 6g
[0059] Tween-80 3.5g
[0060] Magnesium stearate 2g
[0061] Microcrystalline cellulose 6g
[0062] Hydroxypropyl Cellulose 5g
[0063] Preparation Process:
[0064] (1) Azilsartan is micronized, control D90<15 microns, and the prescription amount is weighed;
[0065] (2) Lactose monohydrate is passed through a 60-mesh sieve, and the prescription amount is weighed;
[0066] (3) Cross-linked sodium carboxymethyl cellulose and microcrystalline cellulose are respectively passed through a 60-mesh sieve, and the prescription amount is weighed;
[0067] (4) Mix (1), (2), and (3), then add Tween-80 in equal amounts and mix, pass through a 60-mesh sieve and mix well; after mixing the above raw and auxiliary materials, add hydroxypropyl Soft material made of cellulose; granulated by sieving, dried and granulated, then added with magnesium stearate, mixed evenly, and compressed into table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com