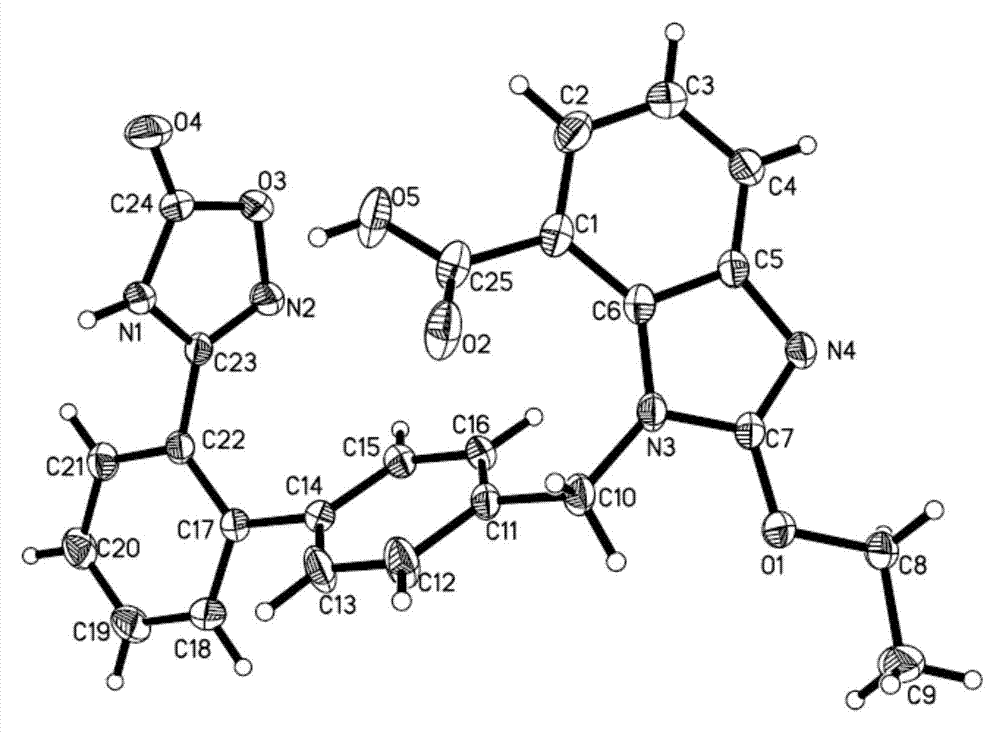
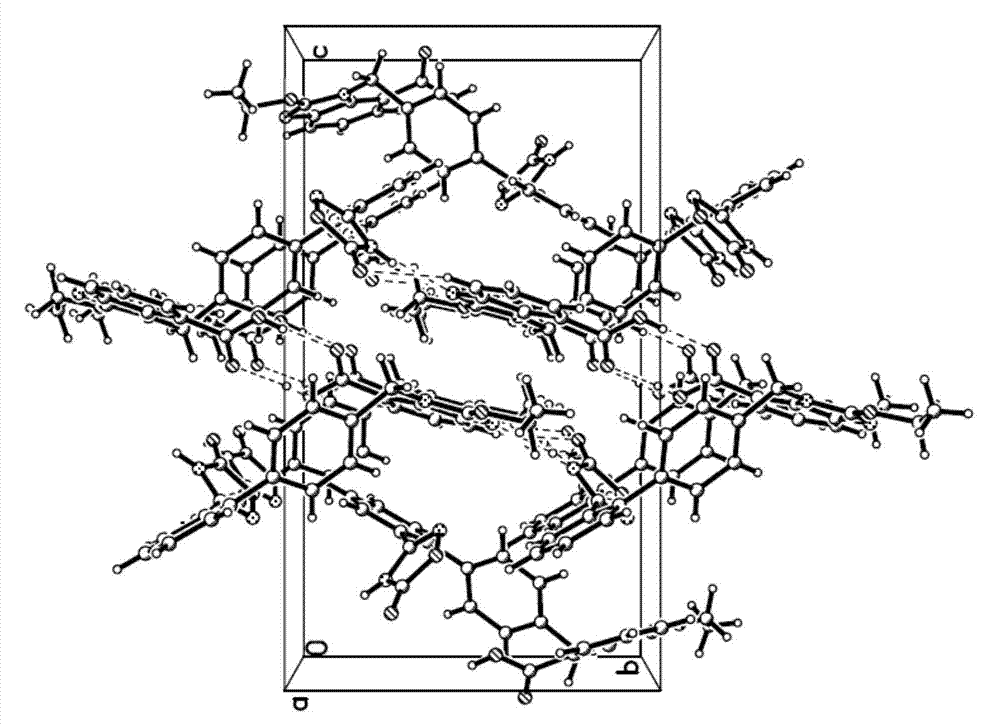
Azilsartan crystal and preparation method thereof
A technology of crystal and unit cell volume, which is applied in the field of medicine and achieves the effects of satisfying large-scale production, high crystal purity and yield, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The preparation method of Azilsartan crystal is as follows:
[0014] In a 100mL beaker, add 50mg of azilsartan, add 50mL of anhydrous methanol, heat until there is reflux on the cup wall, stop heating after the azilsartan is completely dissolved, add anhydrous methanol to the system to 50mL, cover with plastic wrap Seal the beaker, leave a small hole to slowly volatilize the solvent, place the flask in a stable environment, and let it stand at 20°C to slowly volatilize the solvent. After 2 days, a colorless block crystal grows, and the crystal After the growth is complete, filter, wash with anhydrous methanol, and dry to obtain 44.6 mg of a crystal sample with a purity of 99.68% and a melting point of 208-210°C.
[0015] The proton nuclear magnetic resonance spectrum data of product are as follows:
[0016] H-NMR (400MHz, DMSO-d6): δ1.47 (3H, t, J = 7.0), 4.67 (2H, q, J = 7.0), 5.77 (2H, s), 7.07-7.70 (11H, m) .
[0017] Wherein, the chemical shift δ=1.47(3H) and 4.7...
Embodiment 2
[0026] Azilsartan crystal culture method comparative examples are as follows:
[0027] In a 100mL beaker, add 50mg of azilsartan, add 50mL of ethyl acetate, heat until there is reflux on the wall of the beaker, stop heating after the azilsartan is completely dissolved, add ethyl acetate to the system to 50mL, cover with plastic wrap Seal the beaker, leave small holes to slowly volatilize the solvent, place the flask in a stable environment, and let it stand at 20°C to slowly volatilize the solvent. After 2 days, a white crystalline solid precipitates out, and the crystal growth is complete. Afterwards, filter, wash with ethyl acetate, and dry to obtain 43.7mg of crystal samples with a purity of 99.54% and a melting point of 208-211°C, but regular crystals that can be tested by X-ray single crystal diffraction were not obtained.
Embodiment 3
[0029] Azilsartan crystal culture method comparative examples are as follows:
[0030] In a 100mL beaker, add 50mg of azilsartan, add 50mL of absolute ethanol, heat until there is reflux on the wall of the beaker, stop heating after the azilsartan is completely dissolved, add absolute ethanol to the system to 50mL, cover with plastic wrap Seal the beaker, leave small holes to slowly volatilize the solvent, place the flask in a stable environment, and let it stand at 20°C to slowly volatilize the solvent. After 2 days, a white crystalline solid precipitates out, and the crystal growth is complete. Afterwards, filter, wash with absolute ethanol, and dry to obtain 43.9 mg of a crystal sample with a purity of 99.61% and a melting point of 208-209° C., but regular crystals that can be tested by X-ray single crystal diffraction were not obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com