Azilsartan intermediate and preparation method thereof
An intermediate and ethoxy technology, applied in the field of medicine, can solve the problems of difficult purification, complicated operation, poor solubility, etc., and achieve the effects of improving purity and yield, improving production efficiency, and simple reaction process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
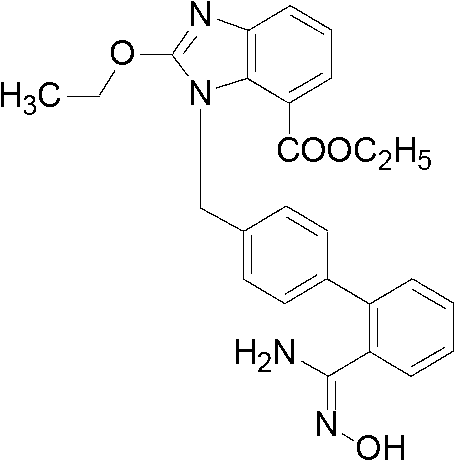
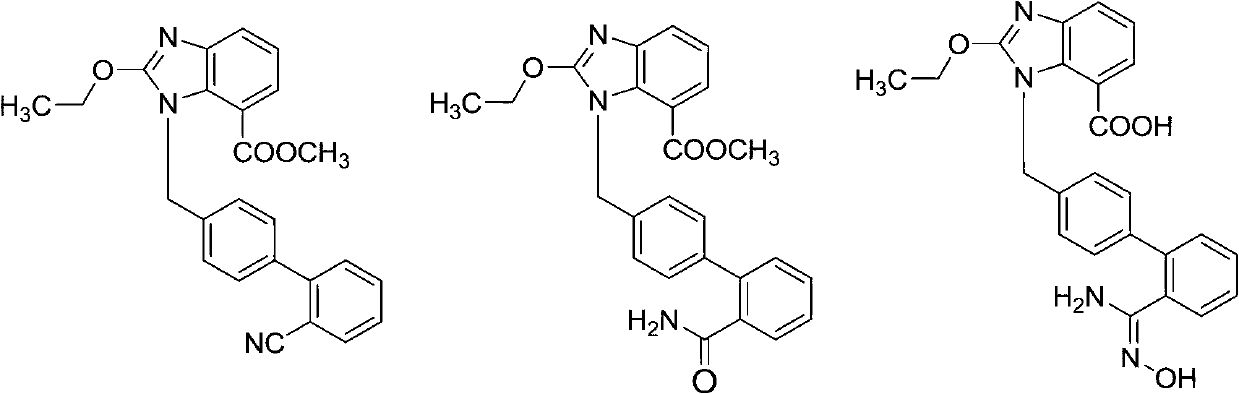
[0031] 2-Ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylic acid ethyl ester (0.50g, 1.18mmol), 50% hydroxylamine Aqueous solution (0.39g, 5.88mmol) in dimethyl sulfoxide (5mL), reacted at 90°C for 8 hours, cooled down, added 12.5mL of water, filtered to obtain 0.47g of the target product, yield 87.23%, HPLC self-normalized purity 93.26 %.
[0032] The above target product 2-ethoxy-1-[[2'-(N'-hydroxyaminomethylimino)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylic acid ethyl ester NMR H spectrum and C spectrum are as follows:
[0033] 1 H-NMR (400MHz, DMSO-d 6 )δ1.18(3H,t),1.42(3H,t),4.22(2H,q),4.62(2H,q),5.54(2H,s),5.56(2H,s),6.94(2H,d ), 7.19(1H,t), 7.28(1H,d), 7.34-7.48(6H,m), 7.70(1H,d), 9.21(1H,s).
[0034] 13 C-NMR (400MHz, DMSO-d 6 )δ13.94,14.41,46.22,61.07,66.63,115.91,120.78,121.49,122.86,125.93,126.92,128.56,128.86,129.83,130.04,130.80,133.21,135.56,139.79,139.85,141.61,151.95,158.28,165.76 .
[0035] MS (ESI + )m / z459.2[M+H] +
Embodiment 2
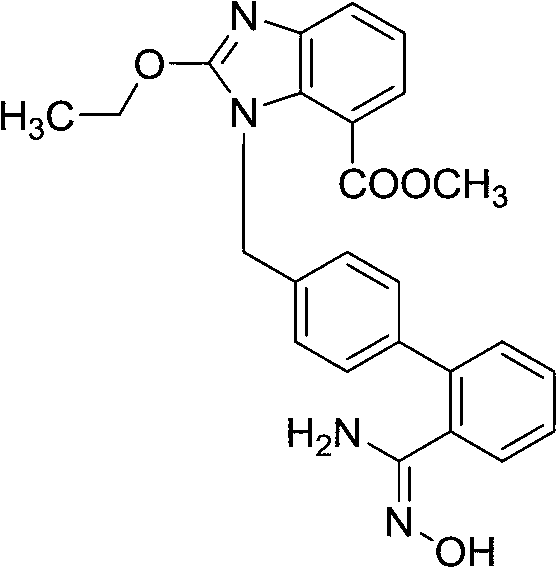
[0037] 2-Ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylic acid ethyl ester (1.00g, 2.35mmol), 50% aqueous solution of hydroxylamine (0.78g, 11.82mmol) in ethanol (10mL), reflux for 24 hours, lower the temperature, add 25mL of water, filter to obtain the target product 0.90g, yield 83.52%, HPLC self-normalized purity 83.32%.
Embodiment 3
[0039] 2-Ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylic acid ethyl ester (1.01g, 2.37mmol), 50% aqueous solution of hydroxylamine (0.94g, 14.24mmol) in ethanol (10mL), reflux for 24 hours, cool down, add 25mL of water, filter to obtain the target product 0.89g, yield 81.77%, HPLC self-normalized purity 80.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com