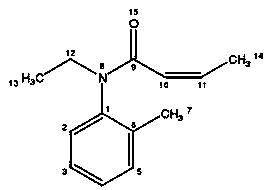
Patents
Literature
35results about How to "Advantages and Significant Advancements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel graded targeted nanoparticles for mediating phototherapy and preparation method and application of novel graded targeted nanoparticles
ActiveCN111297830AAdvantages and Significant AdvancementsImprove stabilityPeptide/protein ingredientsEnergy modified materialsTumor cellsCell
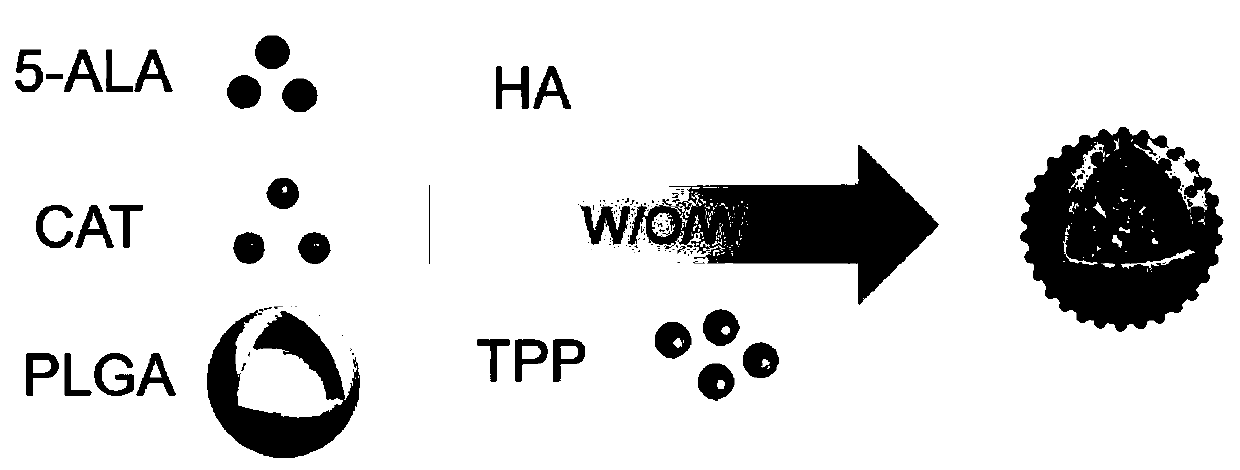
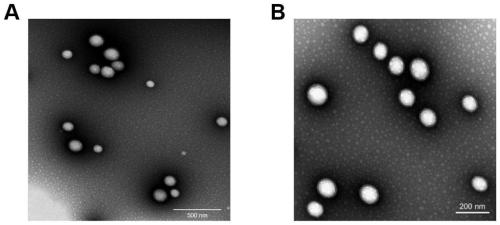
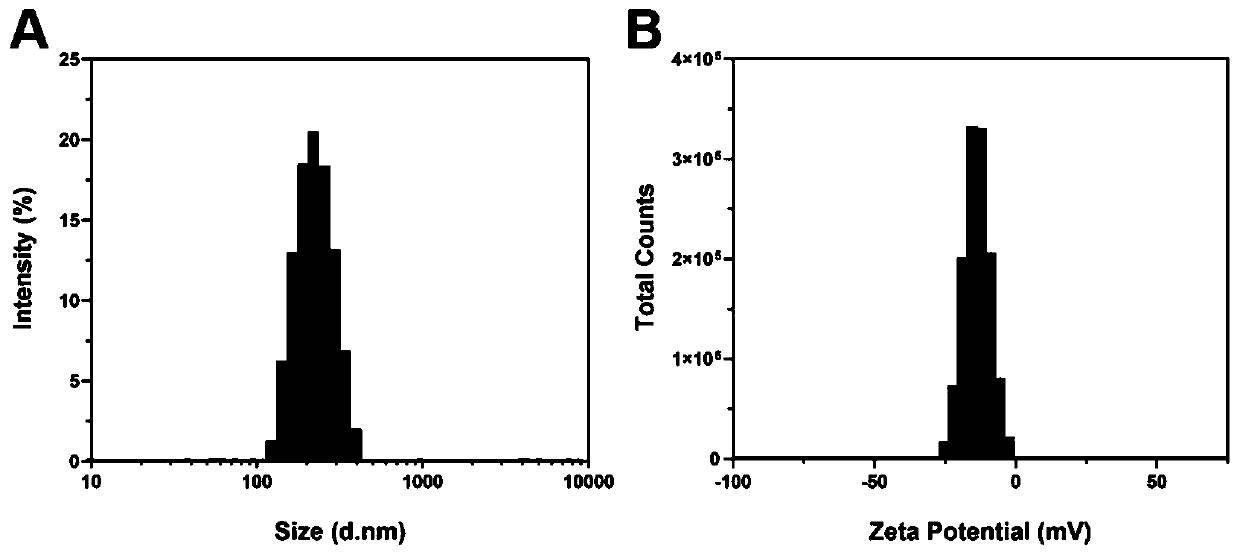
The invention discloses novel graded targeted nanoparticles for mediating phototherapy and a preparation method and application of the novel graded targeted nanoparticles. The targeted nanoparticles are of a core-shell-shell spherical structure, and is prepared by adopting a W / O / W double emulsification method; and 5-ALA and CAT are distributed inside the nanoparticles as a water phase, TPP and PLGA are taken as an oil phase to form the inner shells of the nanoparticles, and the outermost layer is coated with HA to be taken as the outer shells. The nanoparticles can actively target tumor cellsand efficiently deliver a photosensitizer 5-ALA; in addition, the nanoparticles can effectively alleviate the TME hypoxia by further targeting mitochondria in the tumor cells and generating a large amount of oxygen through CAT, and can produce a large amount of ROS and enhance the killing effect of ROS under laser irradiation, so that an enhanced anti-tumor effect is exerted.
Owner:THE AFFILIATED HOSPITAL OF XUZHOU MEDICAL UNIV
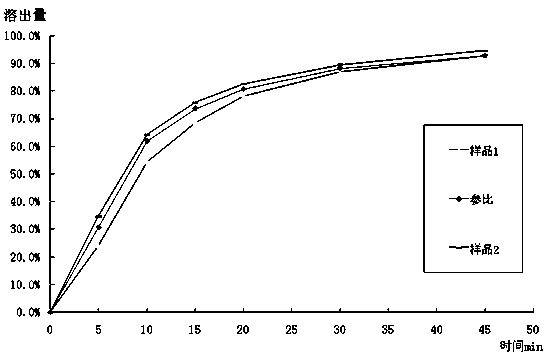
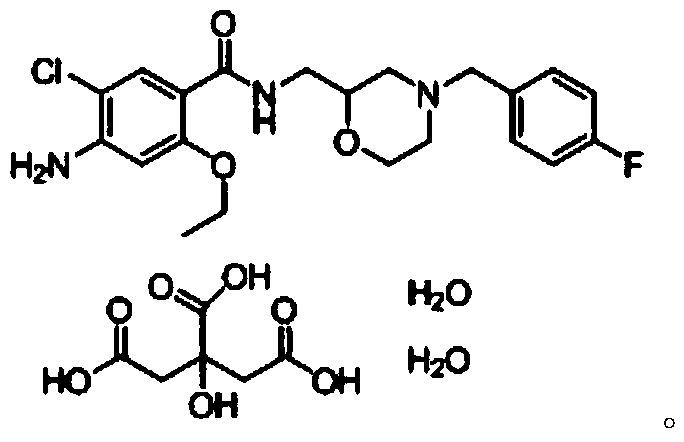
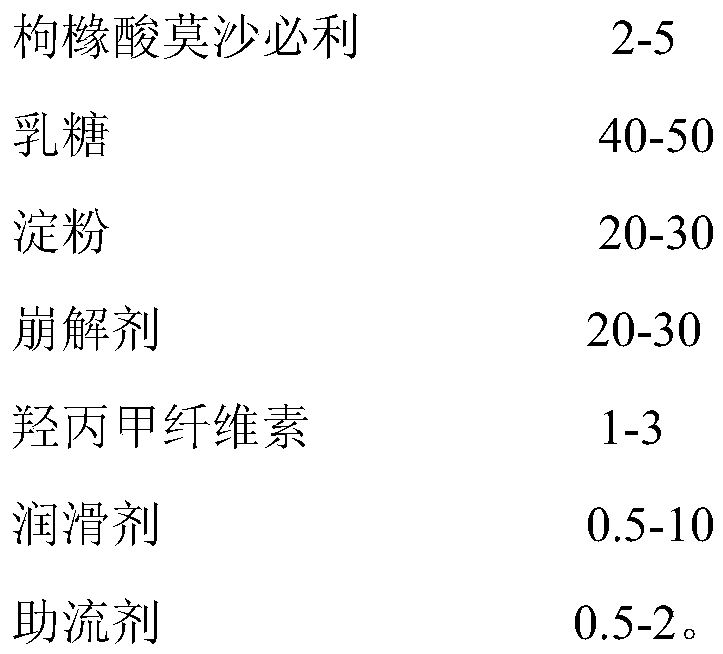
Mosapride citrate tablet and preparation method thereof
ActiveCN104622826AAdvantages and Significant AdvancementsPromote dissolutionOrganic active ingredientsDigestive systemMedicineTableting
The invention discloses a mosapride citrate tablet and a preparation method thereof. The tablet is obtained by virtue of wet granulation and tableting; a binder adopted for wet granulation is the aqueous solution of mosapride citrate and povidone K15. Compared with the prior art, the mosapride citrate tablet is quick in drug dissolution, stable in quality, simple in preparation process without complex preparation equipment, and prone to industrial mass production.
Owner:SHANGHAI PUKANG PHARMA
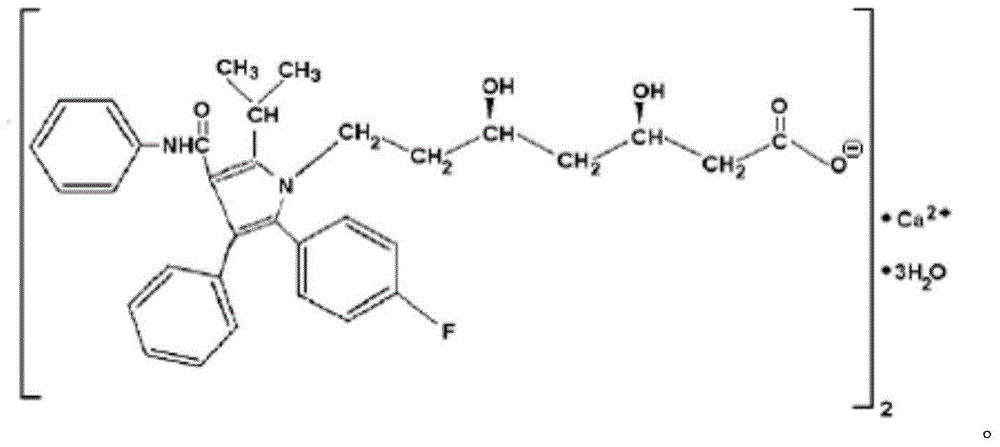
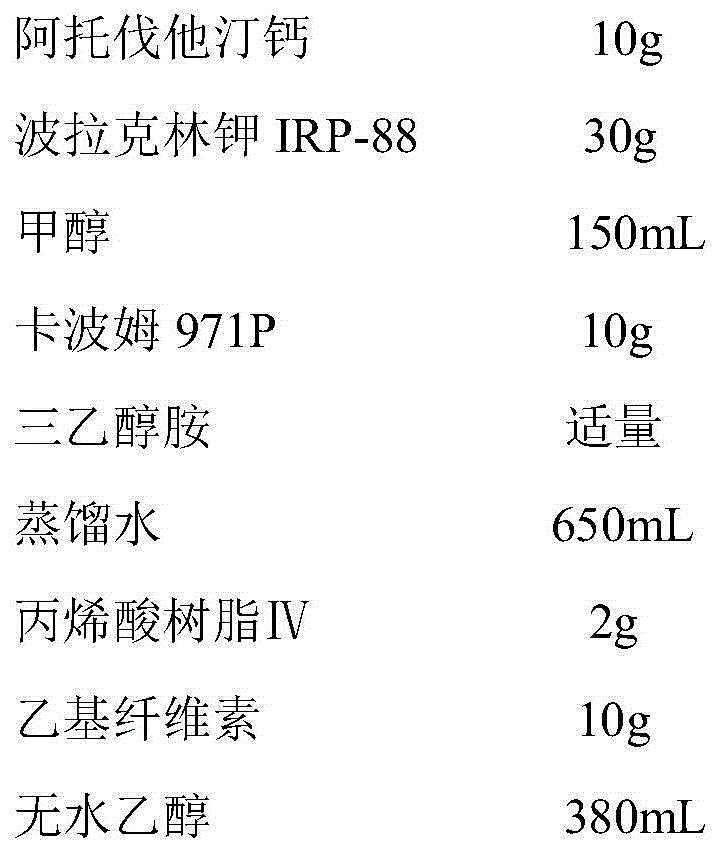
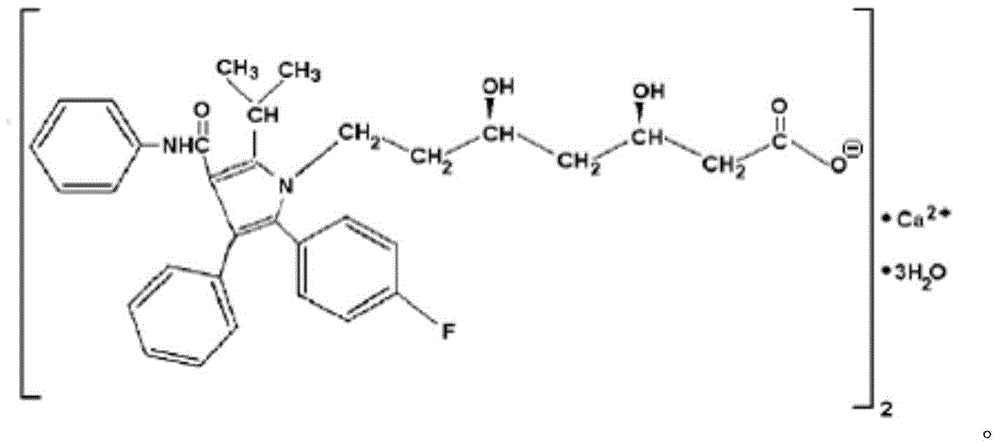
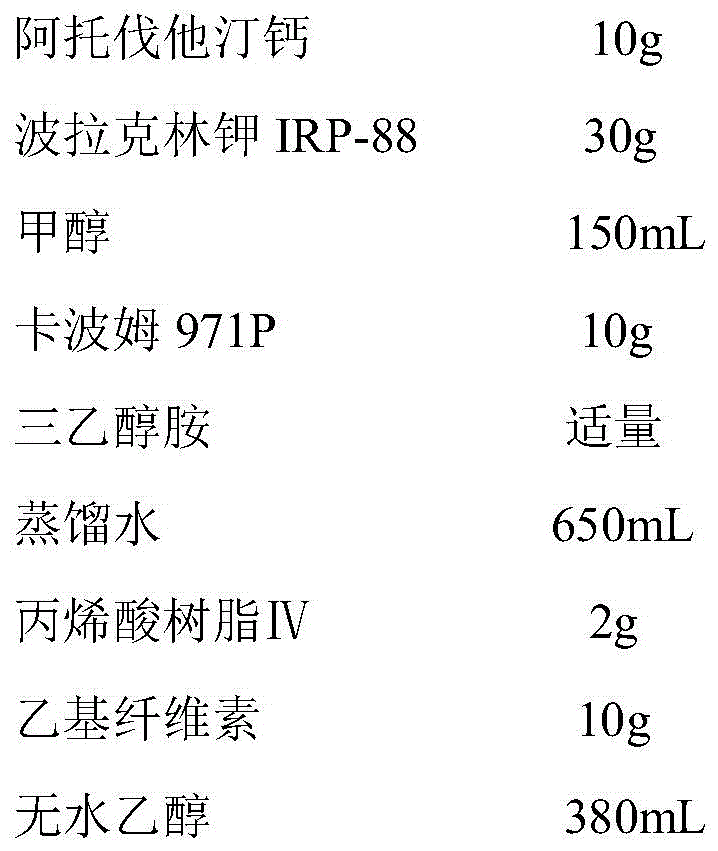
Atorvastatin calcium tablet and preparation method thereof
ActiveCN104306343AAdvantages and Significant AdvancementsFix stability issuesMetabolism disorderPharmaceutical non-active ingredientsSolubilityAcrylic resin
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet is prepared from atorvastatin calcium, polacrilin potassium IRP-88, carbomer 971P, acrylic resin IV, ethyl cellulose and other pharmaceutically acceptable auxiliaries. According to the atorvastatin calcium tablet, the stability problem of atorvastatin calcium preparation and storage processes can be successfully solved, the atorvastatin calcium solid dispersion is prepared by adopting a solvent deposition technology, and the drug solubility can be greatly improved; and stomach discomfort and other side effects can be reduced by means of a semipermeable membrane coating.
Owner:NANJING CHIA TAI TIANQING PHARMA
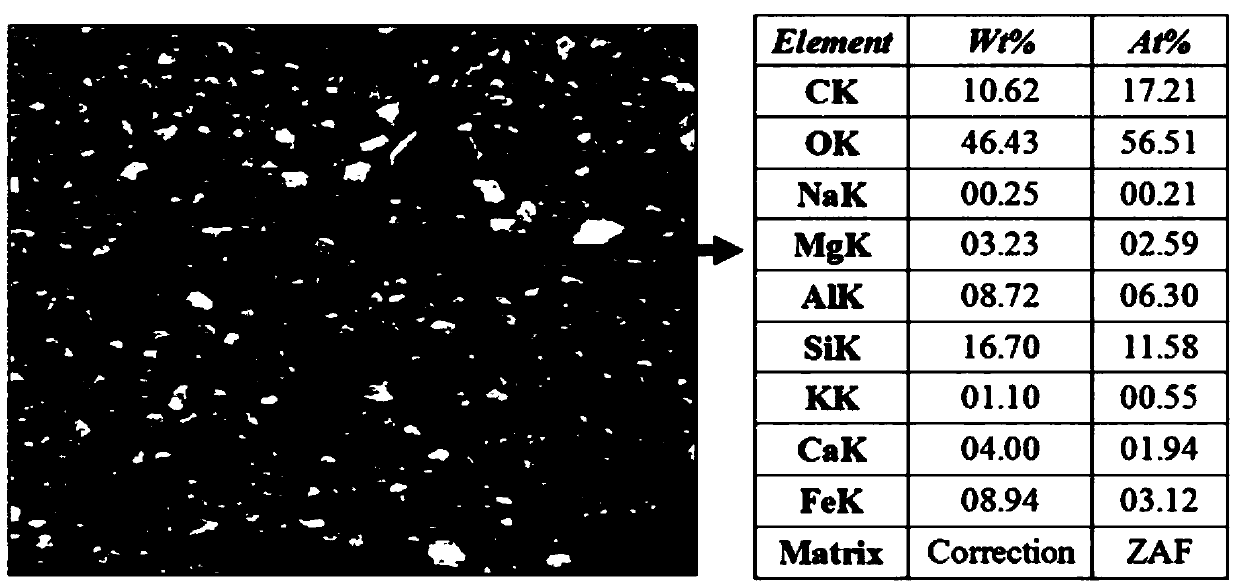
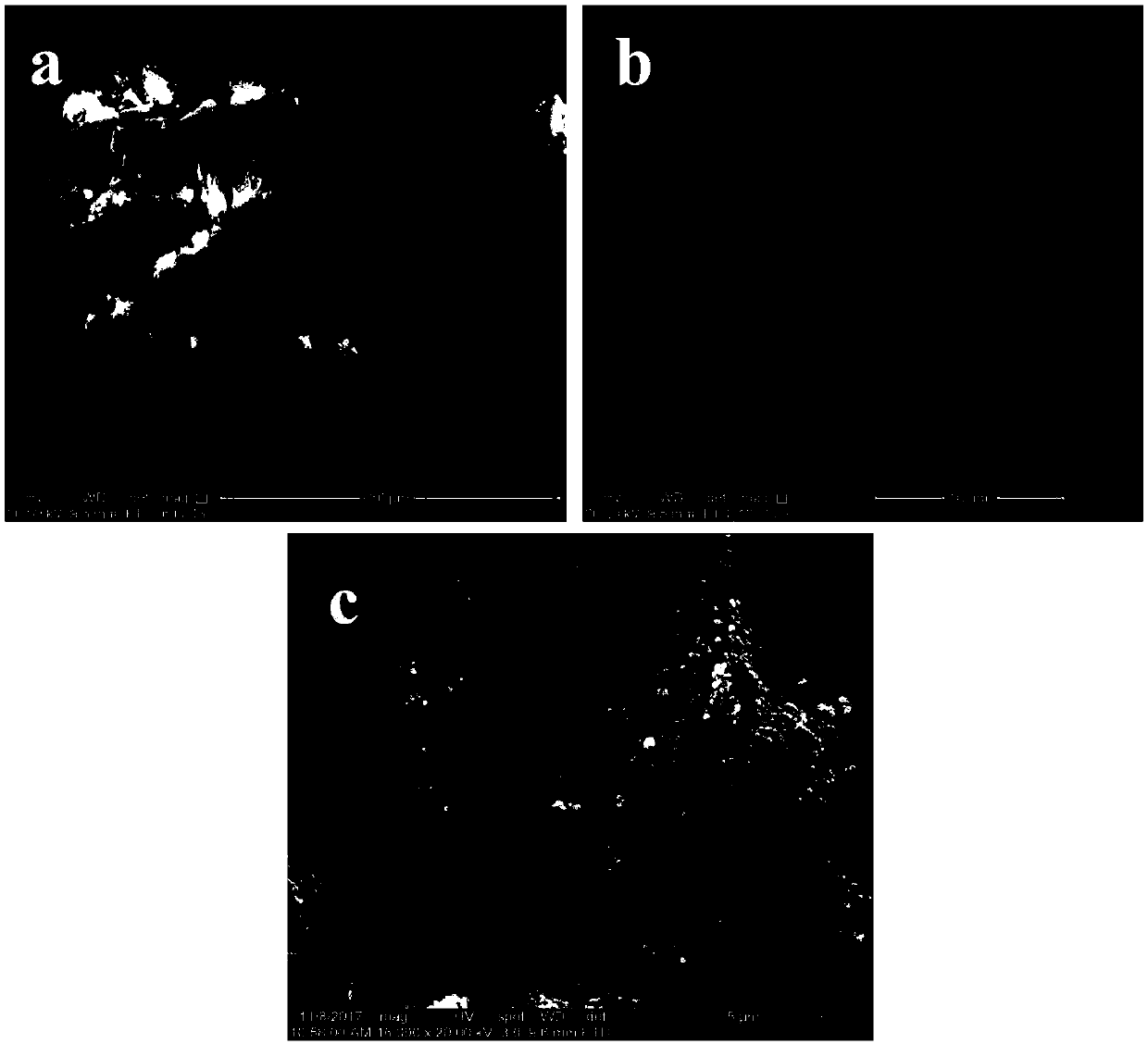
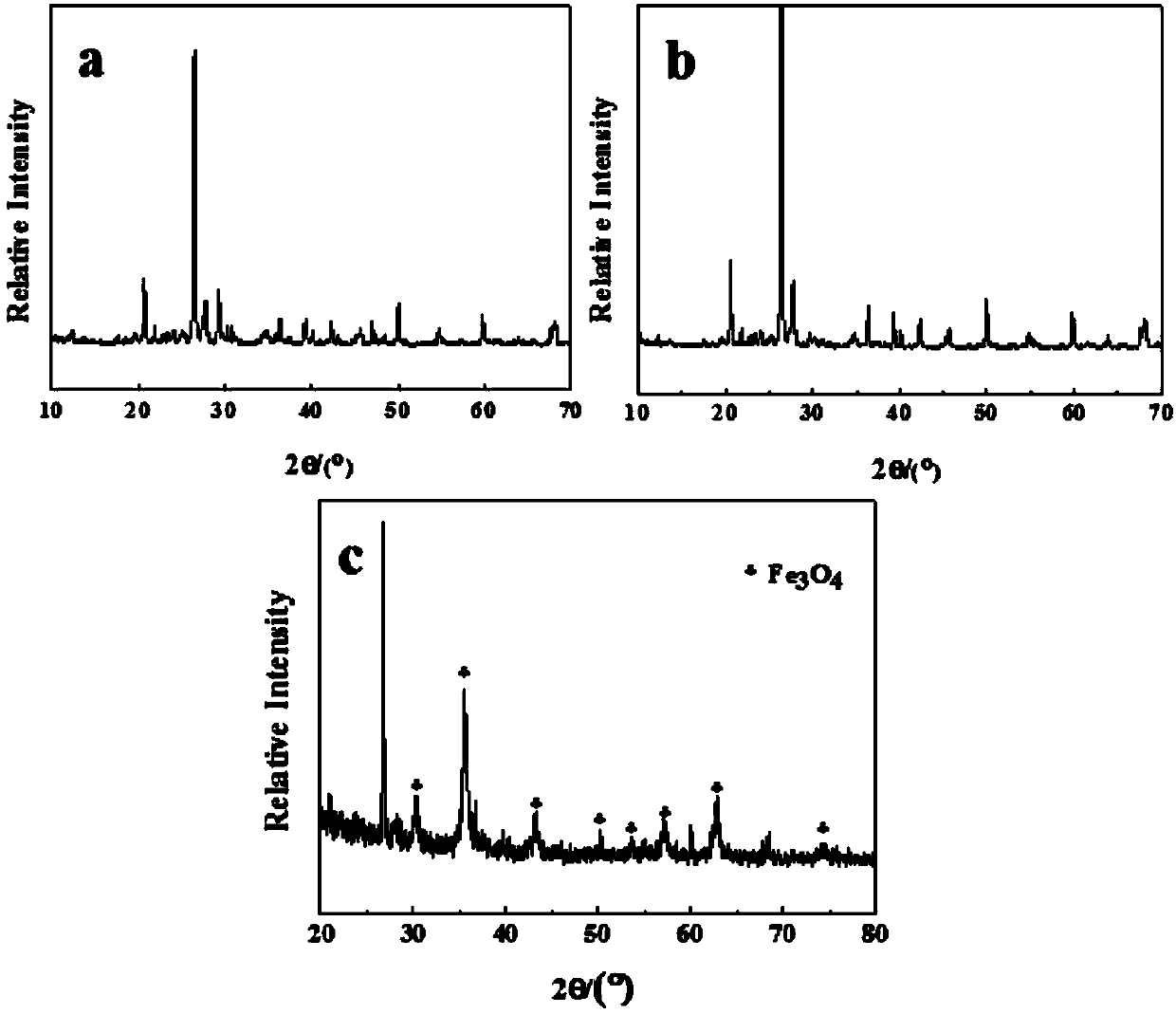
Preparation method and applications of magnetically modified loess adsorbent
InactiveCN107890849ALarge specific surface areaImprove adsorption performanceOther chemical processesWater contaminantsSolventSorbent
The invention provides a preparation method and applications of a magnetically modified loess adsorbent. According to the preparation method, a soluble ferric salt is mixed with an alkali, and solvothermal method is adopted for synthesis so as to obtain nanometer magnetic Fe3O4; and the obtained nanometer magnetic Fe3O4 and activated loess are mixed and then are reacted at sealed conditions at 150to 200 DEG C so as to obtain the magnetically modified loess adsorbent. The removing rate of the magnetically modified loess adsorbent on methylene blue is 90% or higher; after adsorption, separationfrom water body is convenient to realize because of the magnetic properties. The raw materials are easily available and cheap; the preparation method is simple; cost is low; and the preparation method is beneficial for industrialized production.
Owner:BAOJI UNIV OF ARTS & SCI
A kind of Azilsartan tablet and preparation process thereof
ActiveCN104306344BAdvantages and Significant AdvancementsUniform contentOrganic active ingredientsPill deliveryGranularityAzilsartan
The invention discloses azilsartan tablets and a preparation process thereof. The tablets comprise azilsartan, microcrystalline cellulose KG1000 and other pharmaceutically acceptable auxiliary materials and are prepared by pressing by adopting a dry-process direct tabletting process. According to the preparation, the content of azilsartan is uniform, and drugs are rapidly dissolved out within 5 minutes. In addition, the preparation process of the preparation is simple, raw materials are not needed to be specially crushed, the granularity requirement is not strict, complex preparation equipment is not needed, and large-scale industrial production is easily realized.
Owner:NANJING CHIA TAI TIANQING PHARMA
A kind of rivaroxaban tablet for prevention and treatment of embolism disease and preparation method thereof
ActiveCN105030703BAdvantages and Significant AdvancementsPromote dissolutionOrganic active ingredientsPill deliveryDiseaseRivaroxaban
The invention discloses rivaroxaban tablets for preventing and treating embolismic diseases and a preparing method thereof. The tablets are made by tableting a mixture of pharmaceutical-containing granules and other pharmaceutically acceptable auxiliaries; the pharmaceutical-containing granules contain rivaroxaban, span-80, polyoxyethylene hydrogenated castor oil and kaolin. The rivaroxaban tablets dissolve out fast, higher than 90% in 5min, and meanwhile, dissolution stability is good, and a preparing process is simple.
Owner:SHANGHAI XUDONG HAIPU PHARMA
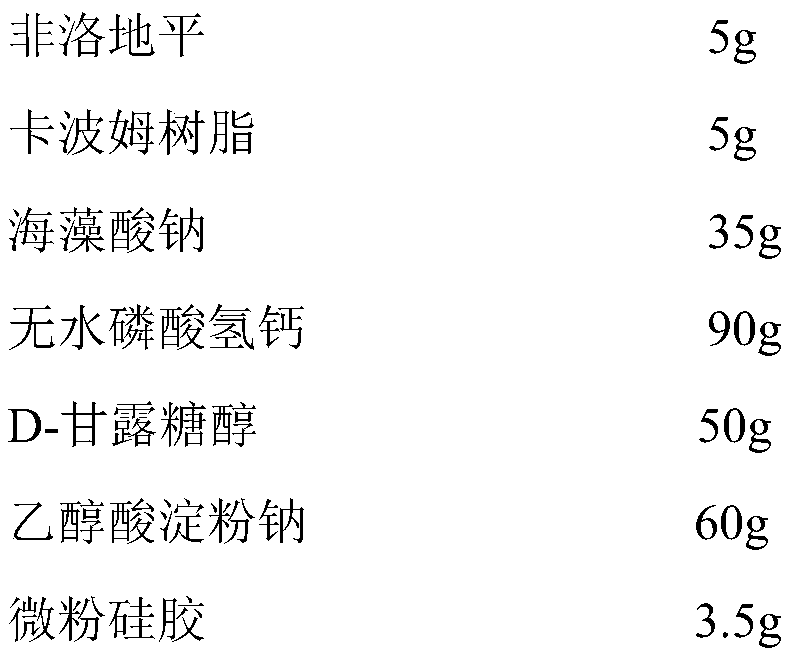
Felodipine sustained release tablet and preparation technology thereof
ActiveCN107550881AAdvantages and Significant AdvancementsAddress release stabilityOrganic active ingredientsInorganic non-active ingredientsSustained Release TabletAlcohol
The invention discloses a felodipine sustained release tablet and a preparation technology thereof and belongs to the field of chemical drug preparations. The felodipine sustained release tablet comprises a felodipine solid dispersion agent, a filling agent, a disintegrating agent and a lubricating agent. The method comprises the following steps: mixing and suspending felodipine, carbomer resin and sodium alginate in absolute ethyl alcohol, drying and removing ethyl alcohol, thereby acquiring the felodipine solid dispersion agent; uniformly mixing with the filling agent and the disintegratingagent, by taking 60% ethyl alcohol as an adhesive, pelletizing and drying at 45 DEG C, thereby acquiring medicated particles; filtering the medicated particles with a 18-mesh sieve, uniformly mixing with a prescription amount of lubricating agent and preforming. The invention successfully solves the problem of felodipine sustained release; the drugs can be completely released; the effective bloodconcentration can be long-term maintained; the bioavailability is high; the preparation process is simple and is suitable for industrial production.
Owner:南京易亨制药有限公司
A kind of atorvastatin calcium tablet and preparation method thereof
ActiveCN104306343BAdvantages and Significant AdvancementsFix stability issuesMetabolism disorderPharmaceutical non-active ingredientsSolubilitySide effect
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet contains atorvastatin calcium, polacrilin potassium IRP-88, carbomer 971P, acrylic resin IV, and ethyl cellulose and other pharmaceutically acceptable excipients. The present invention successfully solves the stability problem during the preparation and storage of atorvastatin calcium, and prepares the solid dispersion of atorvastatin calcium by using solvent deposition technology, which greatly improves the solubility of the drug; at the same time, through semi-permeable membrane coating, Side effects such as upset stomach are reduced.
Owner:NANJING CHIA TAI TIANQING PHARMA
Metronidazole effervescent tablet for vagina and preparation technology of metronidazole effervescent tablet
InactiveCN104666273AAdvantages and Significant AdvancementsFix stability issuesOrganic active ingredientsInorganic non-active ingredientsEffervescent tabletVagina
The invention discloses a metronidazole effervescent tablet for vagina and a preparation technology of the metronidazole effervescent tablet. The tablet is prepared from a metronidazole tablet core and an alkaline coating layer, wherein the metronidazole tablet core contains an acid component; and the metronidazole tablet core is wrapped with the alkaline coating layer. Compared with the prior art, an acid effervescing agent and an alkaline effervescing agent are effectively separated; the problem of the stability of the metronidazole effervescent tablet in preparation and storage processes is successfully solved; meanwhile, the amount of auxiliary materials is greatly reduced; the tablet weight is reduced; and medication of patients is facilitated.
Owner:尹克春
Crotamiton-containing external preparation and preparation process thereof
InactiveCN103830179AImprove efficacyImprove product qualityOrganic active ingredientsPharmaceutical non-active ingredientsPoloxamerGlyceryl Caprate
The invention discloses a crotamiton-containing external preparation and a preparation process thereof. The external preparation is prepared from the following components in parts by weight: 100 parts of crotamiton, 200-400 parts of diethylene glycol monoethyl ether, 100-200 parts of decanoyl / octanoyl-glycerides and 400-600 parts of poloxamers. The preparation process particularly comprises the following steps: dissolving the crotamiton into the diethylene glycol monoethyl ether; then adding the decanoyl / octanoyl-glycerides and the poloxamers; and stirring for dissolution. The preparation process disclosed by the invention is simple, fundamentally avoids the problem that a medicament is separated from a matrix and is favorable to long-term storage and transportation.
Owner:韩峰
Ionic liquid lubricant for titanium alloy and preparation method and applications thereof
InactiveCN109651434AAdvantages and Significant AdvancementsImprove thermal stabilityGroup 5/15 element organic compoundsSulfonic acids salts preparationWear resistantPhysisorption
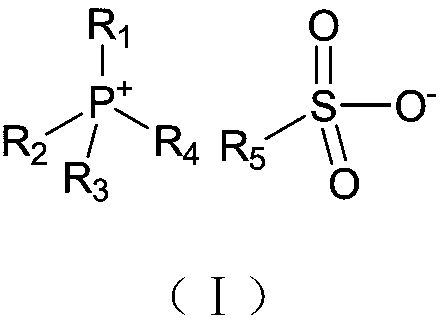
The invention provides an ionic liquid lubricant for titanium alloy and a preparation method and applications thereof. The ionic liquid has a structure represented by the formula (I), in the formula (I), R1, R2, R3 and R4 individually represent a C2-C8 alkyl group and R5 represents a C1-C16 perfluoro-alkyl group. During the friction process, a firm and sequenced physical adsorption protective filmand a chemical reaction protective film are formed on the surface of a metal friction pair; and thus the lubricant has an excellent friction reducing performance and a wear resistant property.
Owner:BAOJI UNIV OF ARTS & SCI
Clopidogrel sulfate tablet and preparation process thereof
ActiveCN104173309AAdvantages and Significant AdvancementsFix stability issuesOrganic active ingredientsPharmaceutical non-active ingredientsHydrogen SulfateCombinatorial chemistry
The invention discloses a clopidogrel sulfate tablet and a preparation process thereof. The tablet contains compound of clopidogrel sulfate and polyvinylpolypyrrolidone, wherein the compound is prepared by the steps of dissolving clopidogrel sulfate in an alcohol solvent, adding polyvinylpolypyrrolidone, uniformly mixing and drying by spray. The clopidogrel sulfate and polyvinylpolypyrrolidone compound prepared by the process is capable of avoiding adhesion in the producing process and successfully solving the stability problem in clopidogrel sulfate preparing and storing processes, is simple in process, and is easy for industrial large-scale production.
Owner:南京康川济医药科技有限公司
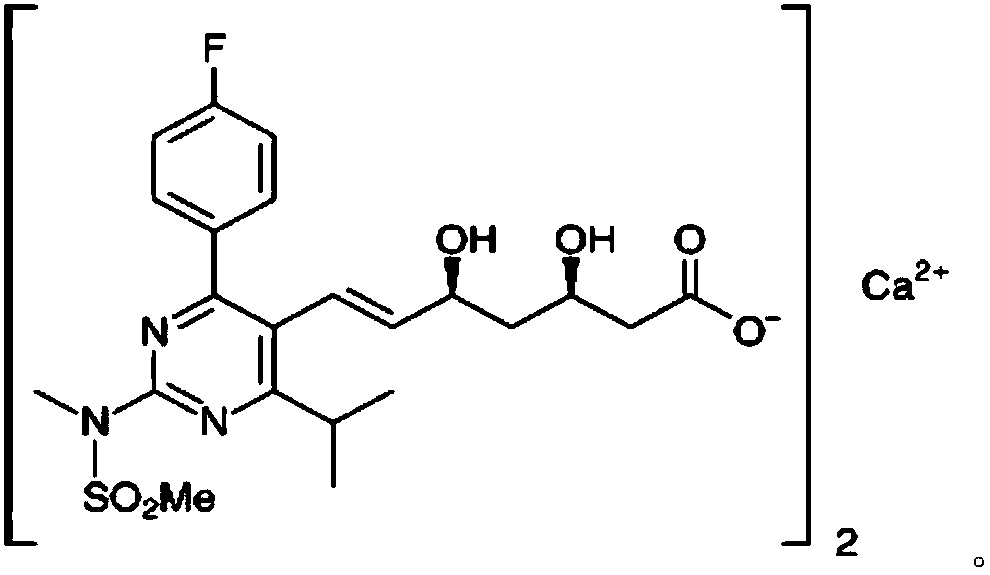
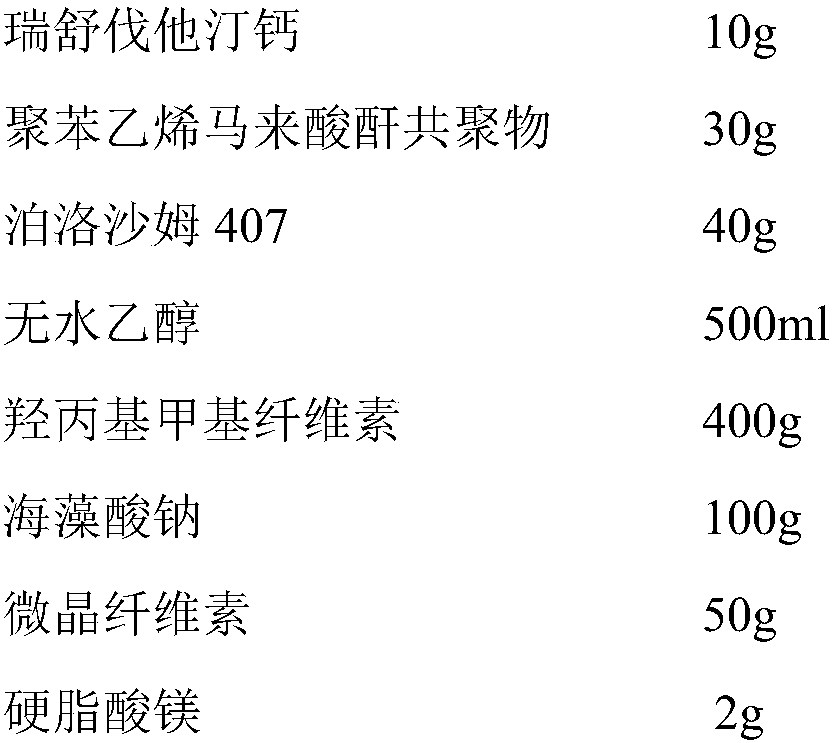
Rosuvastatin calcium tablet and preparation process thereof
InactiveCN104840969AAddress release stabilitySmooth releaseOrganic active ingredientsMetabolism disorderMaleic anhydrideChemistry
The invention discloses a rosuvastatin calcium tablet and a preparation process thereof, and belongs to chemical medicinal preparations. The rosuvastatin calcium tablet is formed by uniformly mixing rosuvastatin calcium solid dispersions with pharmaceutically-acceptable accessories and then carrying out direct compression, the rosuvastatin calcium solid dispersions are obtained by suspending rosuvastatin calcium, a polystyrene maleic anhydride copolymer and poloxamer 407 in absolute ethyl alcohol and drying to remove ethyl alcohol, the rosuvastatin calcium solid dispersions are uniformly mixed with the other pharmaceutically-acceptable accessories, and then the direct compression is carried out. According to the rosuvastatin calcium tablet and the preparation process thereof, the problem of release of the rosuvastatin calcium is successfully solved, and the dissolution stability of the rosuvastatin calcium is remarkably improved.
Owner:KUNMING MEDICAL UNIVERSITY
Preparation method of seasoning peony seed leisure food
ActiveCN113080411AAdvantages and Significant AdvancementsRapid productionFood freezingFood thermal treatmentVANILLA FLAVORINGUltrasound assisted
The invention provides a preparation method of seasoned peony seed leisure food, and belongs to the technical field of deep processing of peony seeds. According to the method, ultrasonic-assisted hot water debitterizing is adopted, a synergistic effect is achieved after an ultrasonic method and a hot water debitterizing method are combined, the debitterizing time is obviously shortened, and industrial rapid production of debitterized peony seeds is facilitated; besides, the debittered peony seeds are deeply processed, so that the peony seed leisure food with three different tastes is provided, and consumers in different regions and at different ages can enjoy the peony seed leisure food.
Owner:HUAZHONG AGRI UNIV +1
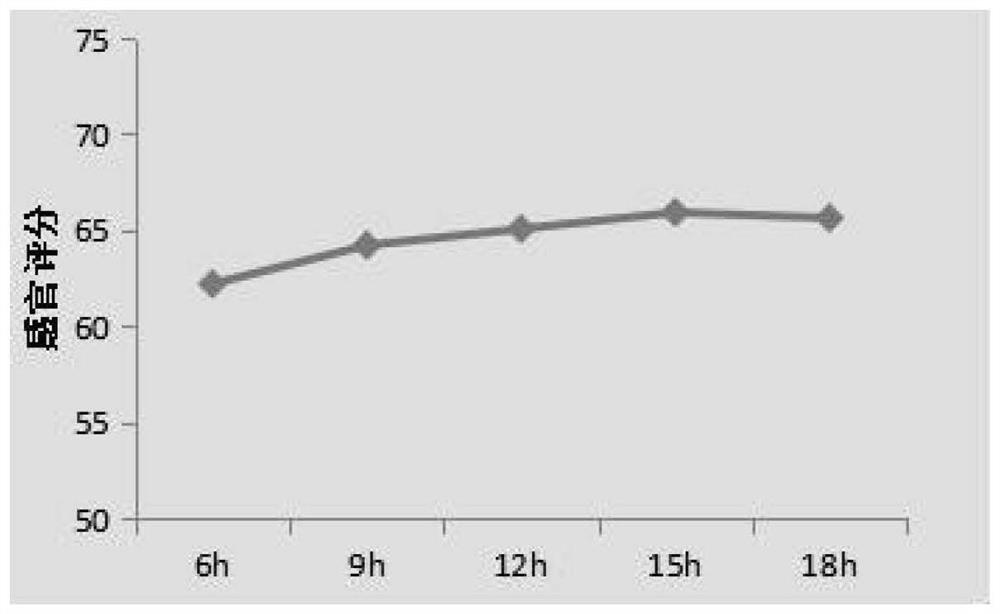
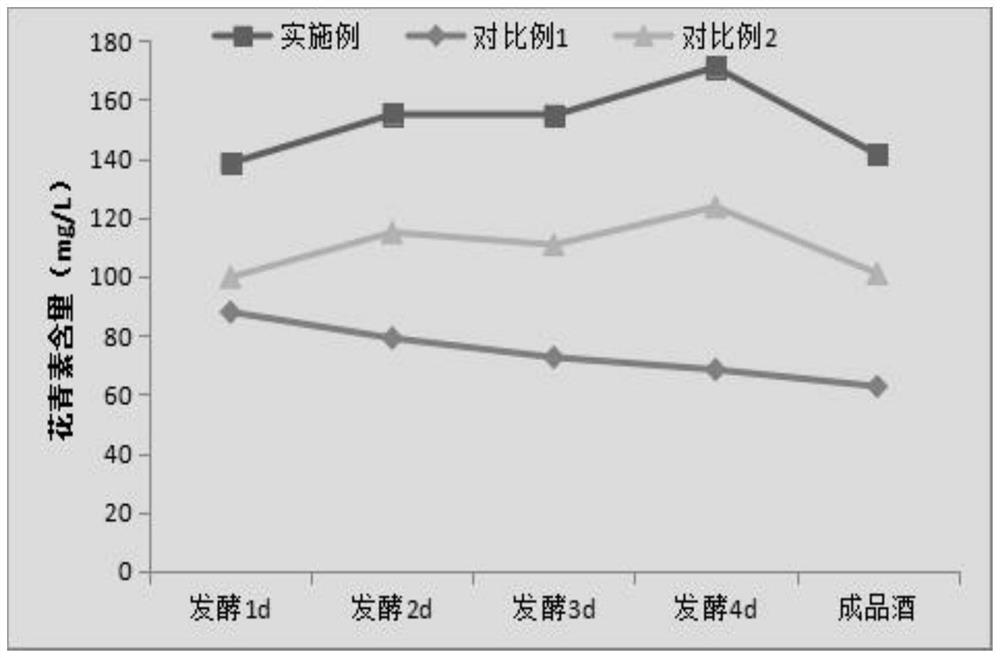
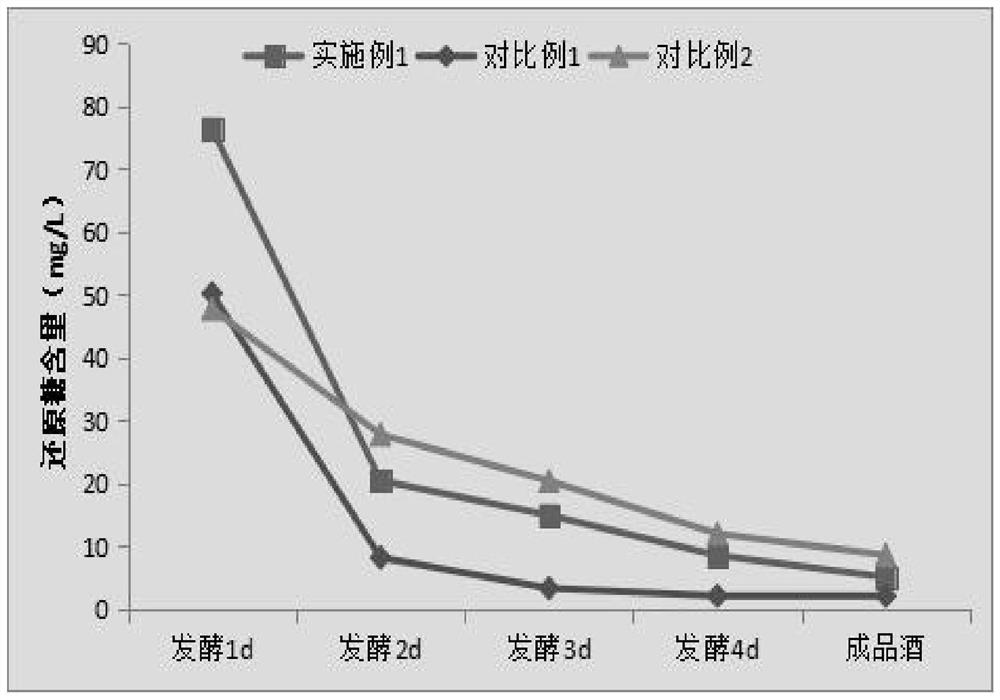
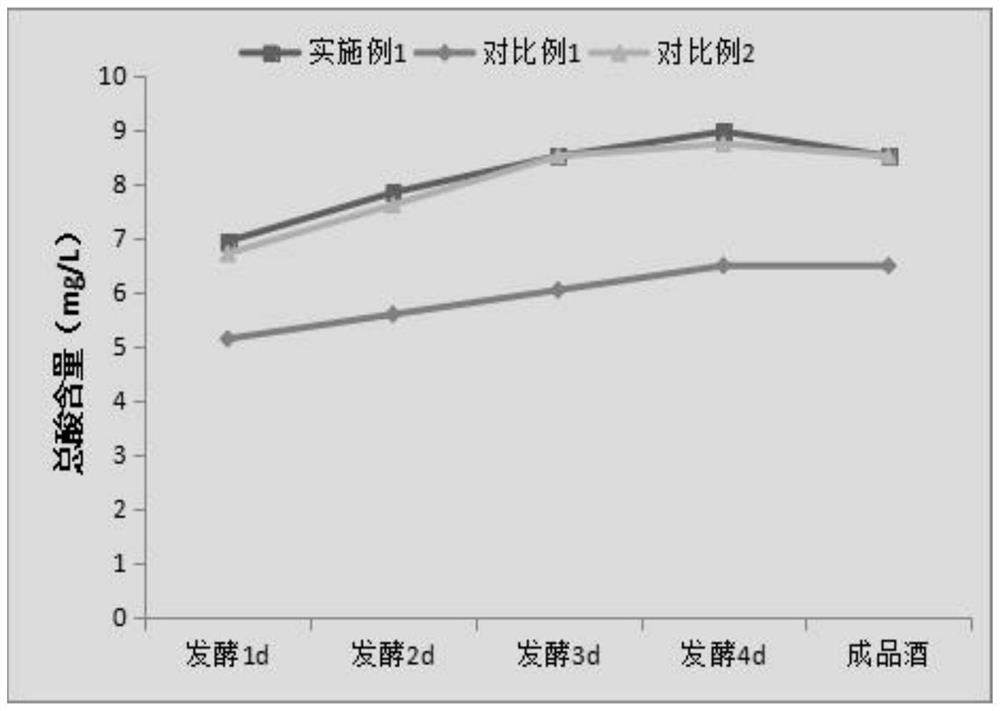
A kind of fully fermented black rice wine brewing method rich in anthocyanin
ActiveCN113355188BAdvantages and Significant AdvancementsHigh in anthocyaninsAlcoholic beverage preparationBiotechnologyBran
The invention belongs to the technical field of black rice wine processing, and provides a brewing method of fully fermented black rice wine rich in anthocyanin. In the method, black rice is properly ground to obtain black polished rice and black rice bran husk. After the black rice bran husk is stabilized by microwave technology, the black polished rice is fermented and the microwave-treated black rice bran husk is added in the alcohol fermentation stage to brew. A fully fermented black rice wine with a dry red wine-like color, mellow taste and unique aroma of black rice is produced. The invention significantly increases the anthocyanin content in the finished wine, improves the quality of the black rice wine, greatly simplifies the production process and saves the production cost, and is beneficial to the realization of mechanization and automation of the factory.
Owner:HUAZHONG AGRI UNIV
A kind of rosuvastatin calcium tablet and preparation technology thereof
InactiveCN104840969BAdvantages and Significant AdvancementsAddress release stabilityOrganic active ingredientsMetabolism disorderPolystyreneDissolution
The invention discloses a rosuvastatin calcium tablet and a preparation method thereof, which belong to the aspect of chemical pharmaceutical preparations and are formed by mixing a rosuvastatin calcium solid dispersion and pharmaceutically acceptable auxiliary materials uniformly and then directly compressing the tablet. The rosuvastatin calcium solid dispersion is made by suspending rosuvastatin calcium, polystyrene maleic anhydride copolymer, and poloxamer 407 in absolute ethanol, drying and removing ethanol to obtain rosuvastatin calcium The solid dispersion is uniformly mixed with other pharmaceutically acceptable auxiliary materials and directly compressed into tablets. The invention successfully solves the release problem of rosuvastatin calcium and significantly improves the dissolution stability of rosuvastatin calcium.
Owner:KUNMING MEDICAL UNIVERSITY
A kind of external preparation containing crotamiton and its preparation process
InactiveCN103830179BAdvantages and Significant AdvancementsImprove efficacyOrganic active ingredientsPharmaceutical non-active ingredientsDiethylene glycol monoethyl etherTopical preparation
The invention discloses a crotamiton-containing external preparation and a preparation process thereof. The external preparation is prepared from the following components in parts by weight: 100 parts of crotamiton, 200-400 parts of diethylene glycol monoethyl ether, 100-200 parts of decanoyl / octanoyl-glycerides and 400-600 parts of poloxamers. The preparation process particularly comprises the following steps: dissolving the crotamiton into the diethylene glycol monoethyl ether; then adding the decanoyl / octanoyl-glycerides and the poloxamers; and stirring for dissolution. The preparation process disclosed by the invention is simple, fundamentally avoids the problem that a medicament is separated from a matrix and is favorable to long-term storage and transportation.
Owner:韩峰
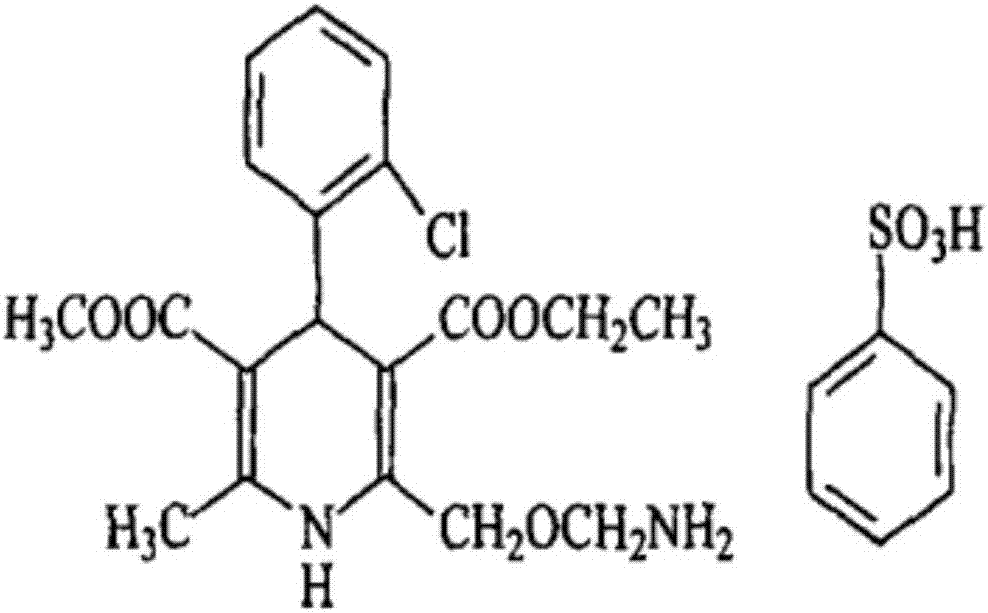
A kind of tablet containing amlodipine besylate L-body or racemate and preparation method thereof
ActiveCN104784137BAdvantages and Significant AdvancementsPromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsAmlodipine besilateDissolution
The invention discloses an amlodipine besylate tablet, wherein the amlodipine besylate is a levorotatory form or a racemic form, and the tablet is prepared by mixing a drug-containing mixture with other pharmaceutically acceptable auxiliary materials. It is directly compressed into tablets after uniformity, and the drug-containing mixture is obtained by mixing amlodipine besylate and copovidone S630, heating until the copovidone S630 melts, and then cooling and solidifying. Compared with the prior art, the preparation of the invention has rapid drug dissolution, uniform content, stable quality, simple preparation process, no complicated preparation equipment, and easy industrialized mass production.
Owner:浙江得恩德制药股份有限公司
A preparation method of flavored peony seed snack food
ActiveCN113080411BAdvantages and Significant AdvancementsRapid productionFood freezingFood thermal treatmentSnack foodVANILLA FLAVORING
The invention provides a preparation method of seasoned peony seed snack food, which belongs to the technical field of deep processing of peony seeds. The method adopts ultrasonic-assisted hot water debittering, and the combination of the ultrasonic method and the hot water debittering method brings a synergistic effect, and the debittering time is obviously shortened, which is beneficial to the rapid industrial production of debittering peony seeds; The bitter peony seeds have been deeply processed to provide three different flavors of peony seed snack foods to satisfy consumers in different regions and age groups.
Owner:HUAZHONG AGRI UNIV +1
Telmisartan hydrochlorothiazide tablet and preparation method thereof
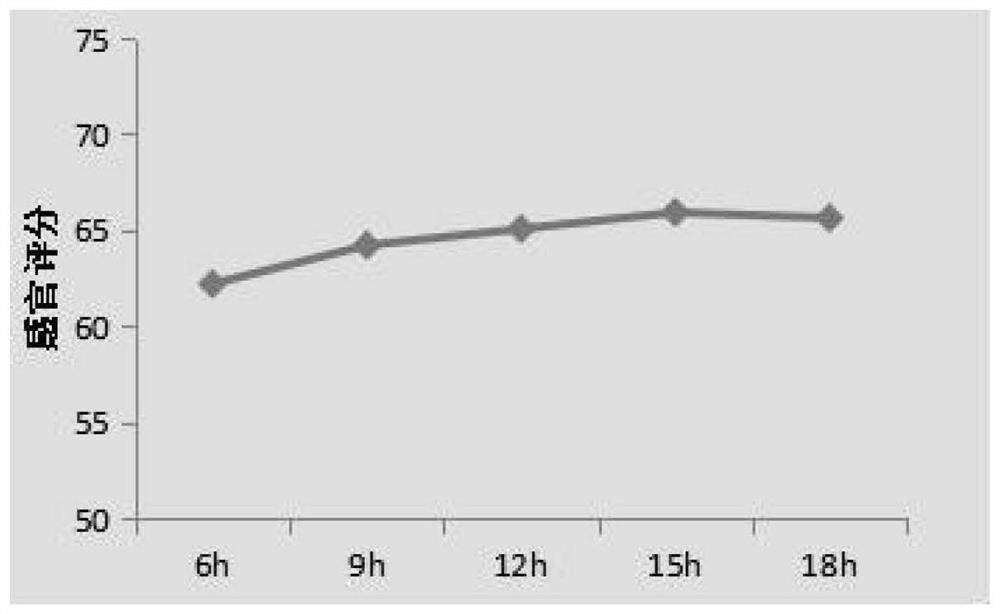
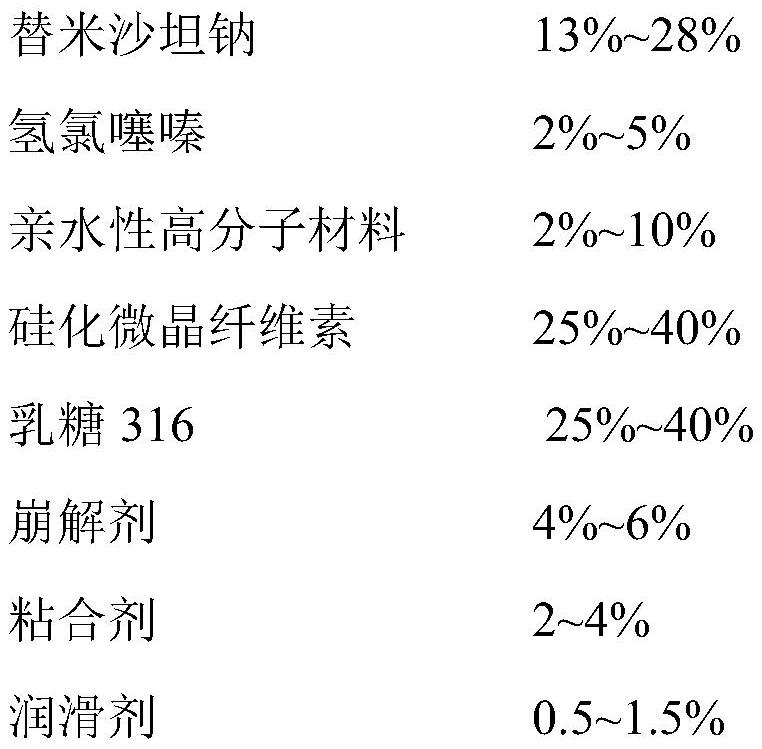
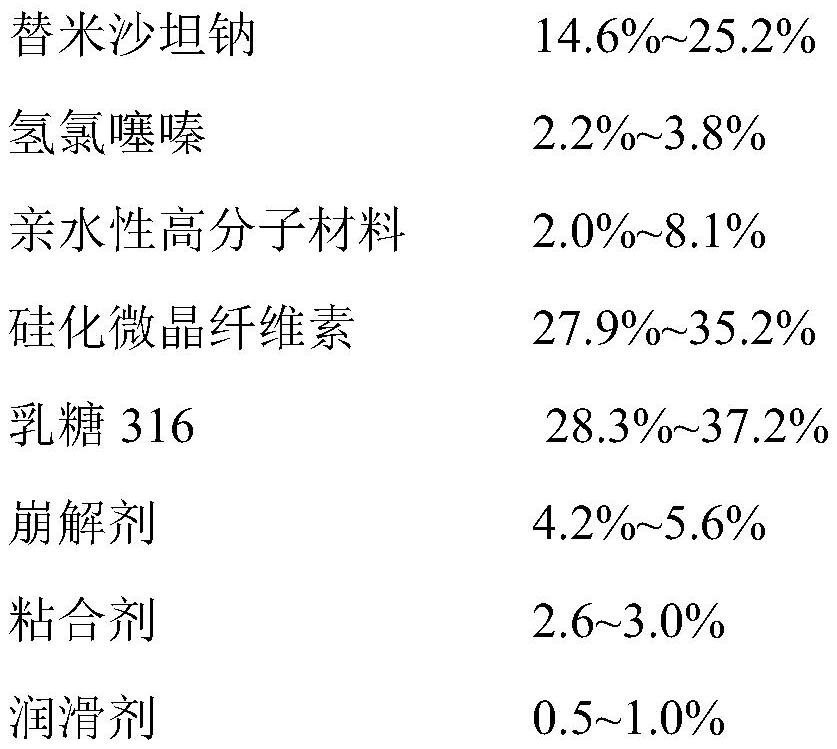
InactiveCN112641745ASimple processEase of industrial productionOrganic active ingredientsPharmaceutical non-active ingredientsTelmisartan/hydrochlorothiazideCellulose
The invention discloses a telmisartan hydrochlorothiazide tablet and a preparation method thereof. The telmisartan hydrochlorothiazide tablet is prepared by coating a drug-containing element tablet, the drug-containing element tablet is prepared by uniformly mixing the following raw materials and auxiliary materials in percentage by mass: 13%-28% of telmisartan sodium, 2%-5% of hydrochlorothiazide, 2%-10% of a hydrophilic polymer material, 25%-40% silicified microcrystalline cellulose, 25%-40% lactose 316, 4%-6% of a disintegrating agent, 2%-4% of an adhesive and 0.5%-1.5% of a lubricant. According to the invention, by adding the hydrophilic polymer material, when the tablet is in an environment with high humidity, the hydrophilic polymer material absorbs water and plays a good role in protecting the raw materials, and besides, the process is simple and is suitable for industrial production.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Mosapride citrate tablet and preparation method thereof
ActiveCN107744509BAdvantages and Significant AdvancementsPromote dissolutionOrganic active ingredientsDigestive systemLactosePharmaceutical Aids
The invention relates to a mosapride citrate tablet and a preparation method thereof, and belongs to the technical field of medical preparations which are applied to medical use and contain organic active ingredients. With mosapride citrate as a raw material and lactose, starch and hydroxypropyl methylcellulose as adjuvant materials, and assisted by aids, the mosapride citrate tablet is prepared by conducting wet-process granulation and tabletting. The mosapride citrate tablet provided by the invention, when applied to the preparation of gastric motor drugs, has the characteristics of being rapid in dissolution, good in stability, simple in preparation process, easy for industrial mass production and the like.
Owner:ZHEJIANG ANGLIKANG PHARMA
A kind of felodipine sustained-release tablet and preparation process thereof
ActiveCN107550881BAdvantages and Significant AdvancementsAddress release stabilityOrganic active ingredientsInorganic non-active ingredientsExtended release tabletsAlcohol
Owner:南京易亨制药有限公司
Compound alpha-ketonic acid tablet and preparation method of same
InactiveCN107875145AHigh capillary activityValid entryPharmaceutical non-active ingredientsUrinary disorderAlcoholKetonic acids
The invention discloses a compound alpha-ketonic acid tablet and a preparation method of same. In the invention, five ketonic acids and five amino acids are subjected to wet-process granulation independently, and then the granules are blended and tabletted, finally the tablets are coated. With organic alcohols as a wetting agent, problems of agglomeration and high viscosity during the granulationare solved, so that security of the medicine is guaranteed. Through specific selection on auxiliary materials, the tablet has excellent dispersibility and binding capability and shows outstanding disintegration capability. The tablet has enough smoothness and hardness and has excellent appearance. By matching the auxiliary materials with the main material, excellent dissolution property of the tablet is guaranteed, and excellent stability during subsequent storage process is achieved. The compound alpha-ketonic acid tablet has excellent appearance, is good in compressibility and dissolubility,has simple and stable processes and is suitable for demands of industrial large-scale production.
Owner:ZHEJIANG ANGLIKANG PHARMA
A kind of ilupolyline enteric-coated tablet and preparation method thereof
ActiveCN105250232BAdvantages and Significant AdvancementsFix stability issuesOrganic active ingredientsDigestive systemSide effectDissolution
The invention discloses an eluxadoline enteric coated tablet and a preparation method thereof. The preparation method includes: mixing eluxadoline with a solubilizer evenly, then dissolving the mixture in a 95% ethanol solution, mixing the substances evenly, then adding an appropriate amount of lecithin, conducting drying at 50DEG C for 45min to obtain dry particles, performing sieving by a 80-mesh sieve, then mixing the product with a filling agent and a disintegrating agent evenly, adding a lubricant tablet, and finally carrying out isolating coating and enteric coating. The eluxadoline enteric coated tablet provided by the invention does not contain basic ingredients, reduces stomach discomfort and other side effects, glyceryl monostearate is added in the enteric coating to significantly improve the dissolution degree of eluxadoline and its stability in gastric juice. Dissolution experiments find that the eluxadoline dissolution degree increases significantly and no burst release phenomenon occurs, the 6h dissolution degree is above 99%, acceleration tests find that the eluxadoline enteric coated tablet has good stability during preparation and storage, the preparation process is simple, has no need for complex preparation equipment, and is easy to realize industrial mass production.
Owner:江阴智产汇知识产权运营有限公司
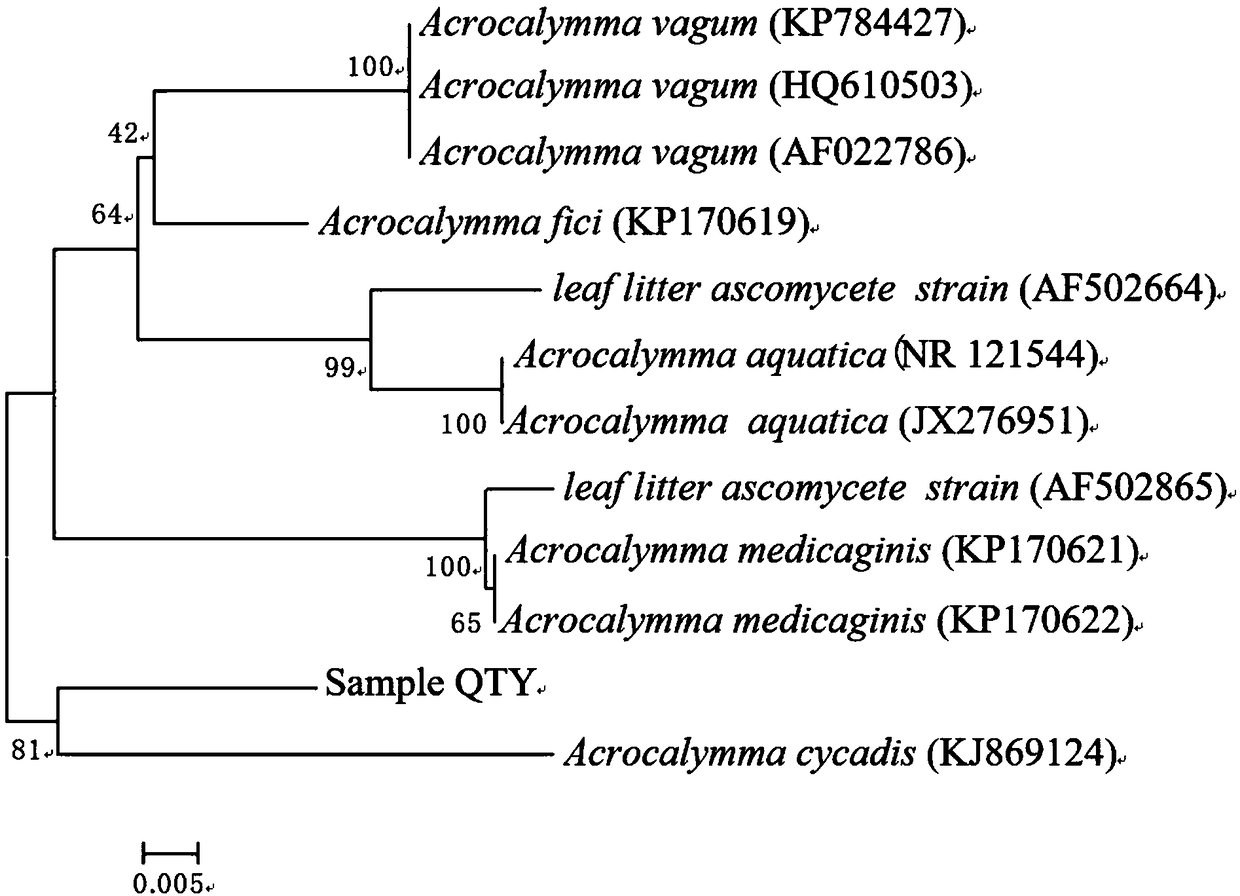
Novel acrocalymma sp. QTY and application thereof in biological control
ActiveCN108192832AAdvantages and Significant AdvancementsGood inhibitory effectBiocideFungiDiseaseSinomenium
The invention discloses application of a strain of novel acrocalymma sp. QTY in the field of biological control. The acrocalymma sp. QTY is obtained by separating from Qinling medicinal plant-sinomenium acutum, and then purifying; the isogeny degree between the acrocalymma sp. QTY and acrocalymma cycadis of morosphaeriaceae is the highest and reaches 94%; the strain provided by the invention is anew strain and is name as acrocalymma sp. QTY. The acrocalymma sp. QTY, is preserved in China Center for Type Culture Collection (CCTCC) on January 25, 2018, and has a preservation number of CCTCC M 2018058. The acrocalymma sp. QTY has a very strong inhibitory effect on apple anthracnose pathogen, apple rot pathogen, wheat gibberellic disease pathogen, tomato gray mold pathogen, tomato early blight pathogen, rice blast pathogen and potato dry rot pathogen, and the relative inhibition rates of the plant pathogens are respectively 100%, 96%, 92%, 100%, 96%, 100% and 100%; therefore, the novel acrocalymma sp. QTY can be applied to the field of biological control and is used for preventing and controlling the plant diseases caused by the pathogens.
Owner:BAOJI UNIV OF ARTS & SCI
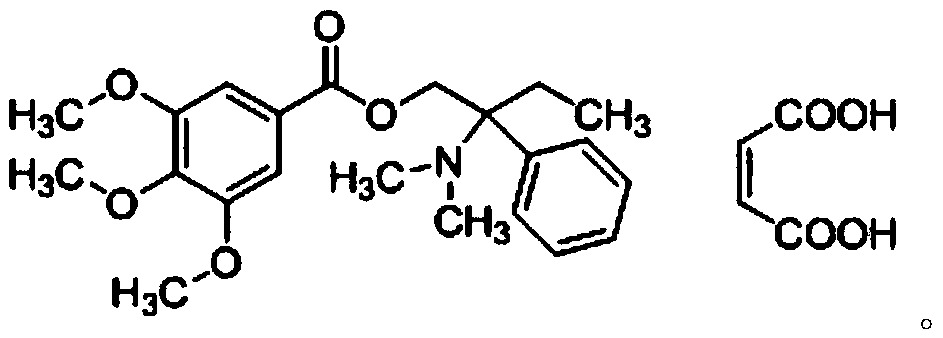
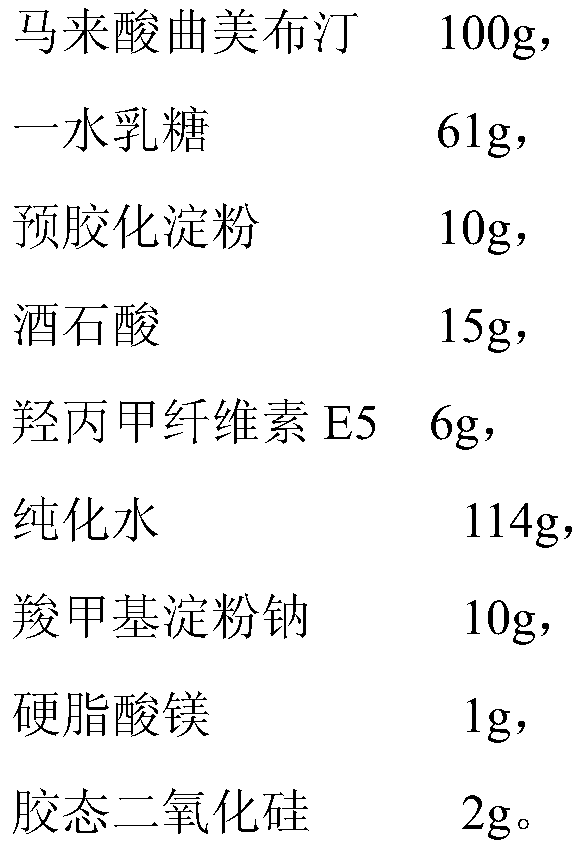
Trimebutine maleate dispersible tablet and preparation method thereof
ActiveCN108078936BAdvantages and Significant AdvancementsPromote dissolutionDigestive systemInorganic non-active ingredientsFunctional disturbanceLactose
The invention relates to a trimebutine maleate dispersible tablet and a preparation method thereof, and belongs to the technical field of medical supplies characterized by used non-effective components. The trimebutine maleate dispersible tablet is prepared from trimebutine maleate, auxiliary materials and additives for adjusting the whole uniformity and dispersibility of the tablet, wherein the auxiliary materials and the additives are added by taking trimebutine maleate as a reference, and the auxiliary materials are prepared from lactose, starch and hypromellose E5. The trimebutine maleatedispersible tablet provided by the invention has the advantages of stable release performance, high bioavailability and the like in different in vitro environments when being applied to processing ofpreparations for conditioning functional disturbances of gastrointestinal tracts.
Owner:ZHEJIANG ANGLIKANG PHARMA
Bendroflumethiazide tablet and preparation method thereof
InactiveCN106943364APromote dissolutionUniform contentOrganic active ingredientsUrinary disorderMagnesium stearatePharmaceutical formulation
The invention discloses a bendroflumethiazide tablet and a preparation method thereof and belongs to the technical field of medicine preparations. The bendroflumethiazide tablet is composed of, by weight, 0.25-0.5 parts of bendroflumethiazide, 2-6 parts of microcrystalline cellulose PH-102, 4-8 parts of lactose, 0.2-0.6 parts of carboxymethyl starch sodium, and 0.02-0.04 parts of magnesium stearate. The preparation method of the bendroflumethiazide tablet includes the steps of: performing air jet pulverization to the bendroflumethiazide; uniformly mixing the bendroflumethiazide, microcrystalline cellulose PH-102 and magnesium stearate to obtain a mixture material; adding the lactose and carboxymethyl starch sodium to the mixture material, and uniformly mixing the materials; and performing dry-method direct tabletting to obtain the bendroflumethiazide tablet. The bendroflumethiazide tablet is more homogenous in content of bendroflumethiazide and is more rapidly to dissolve out in 5 min. The preparation method is simple, wherein the auxiliary materials, except the microcrystalline cellulose PH-102, are free of special pulverization treatment. The preparation method is free of complex preparation devices and is easy to achieve in industrial production.
Owner:HUAYI PHARMA ANHUI CO LTD
A kind of clopyrrole hydrogen sulfate tablet and preparation technology thereof
ActiveCN104173309BAdvantages and Significant AdvancementsFix stability issuesOrganic active ingredientsPharmaceutical non-active ingredientsSulfationAlcohol
The invention discloses a clopidogrel sulfate tablet and a preparation process thereof. The tablet contains compound of clopidogrel sulfate and polyvinylpolypyrrolidone, wherein the compound is prepared by the steps of dissolving clopidogrel sulfate in an alcohol solvent, adding polyvinylpolypyrrolidone, uniformly mixing and drying by spray. The clopidogrel sulfate and polyvinylpolypyrrolidone compound prepared by the process is capable of avoiding adhesion in the producing process and successfully solving the stability problem in clopidogrel sulfate preparing and storing processes, is simple in process, and is easy for industrial large-scale production.
Owner:南京康川济医药科技有限公司
Preparation method of polygonatum sibiricum red-sourced resistant starch containing noodles
ActiveCN109588632AAdvantages and Significant AdvancementsReflect the health functionFood scienceTriticum turgidumPolygonatum sibiricum
The invention discloses a preparation method of polygonatum sibiricum red-sourced resistant starch containing noodles. The preparation method comprises the following steps: preparing polygonatum sibiricum red-sourced resistant starch from polygonatum sibiricum red as a raw material, and jointly processing the polygonatum sibiricum red-sourced resistant starch, a polygonatum sibiricum red starch processing byproduct, namely a polygonatum sibiricum red extract, and wheat flour into high-quality noodles with special active components of the polygonatum sibiricum red. The raw material polygonatumsibiricum red is fully utilized, and the health-care functions of the raw material are embodied; the processed noodles are not only high in quality, low in broken rate, sufficient in elasticity, smallin cooking loss and excellent in palatability; and the problem that the quality of the original wheat-flour noodles is reduced after addition of a large amount of resistant starch is effectively solved.
Owner:FARM PROD PROCESSING & NUCLEAR AGRI TECH INST HUBEI ACAD OF AGRI SCI +1
A kind of pharmaceutical composition and application thereof for accelerating burn and scald wound healing
ActiveCN104000894BQuick and precise wound pain reliefShorter wound healing timePowder deliveryDermatological disorderWound healingWeed
The invention relates to a pharmaceutical composition accelerating healing of burn and scald wounds and the application of the pharmaceutical composition. The pharmaceutical composition comprises, by mass, 50-80 parts of roots of giant knot weeds, 50-80 parts of purslane, 28-40 parts of yellowweed and 15-25 parts of roots of lineate supplejack. When the pharmaceutical composition is applied to treatment of burns and scalds, pain of the wounds is relieved rapidly and definitely, the healing time of the wounds is short, the application method is easy, and a user spends little money.
Owner:NANTONG FINC PHARMA CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com