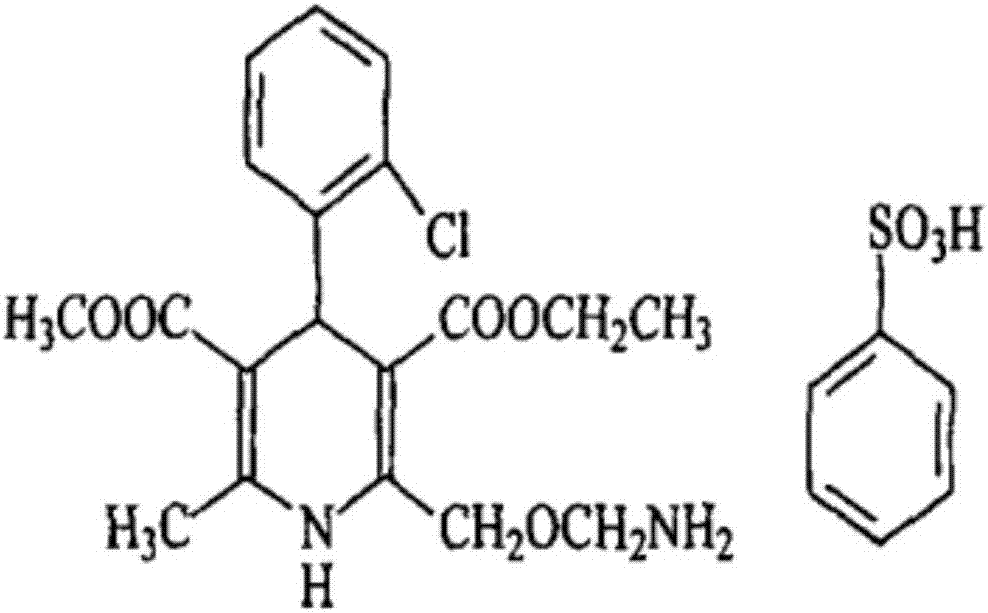
A kind of tablet containing amlodipine besylate L-body or racemate and preparation method thereof
A technology of amlodipine besylate and tablets, which is applied to medical preparations containing active ingredients, medical preparations without active ingredients, pill delivery, etc., can solve problems such as complex preparation processes, and achieve uniform drug content, The particle size requirement is not harsh and the preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]
[0028] Preparation:
[0029] Mix the prescribed amount of amlodipine besylate and copovidone S630 evenly, heat under stirring at 60°C until the copovidone S630 melts, cool to 25°C, and solidify for 10 hours to obtain a drug-containing mixture; After a 60-mesh sieve, it is evenly mixed with lactose, microcrystalline cellulose, crospovidone and magnesium stearate in the prescribed amount, and then compressed into tablets.
Embodiment 2
[0031]
[0032] Preparation:
[0033] Mix the prescribed amount of amlodipine besylate and copovidone S630 evenly, heat under stirring at 63°C until the copovidone S630 melts, cool to 20°C, and solidify for 9 hours to obtain a drug-containing mixture; After the 50-mesh sieve, it is evenly mixed with the prescribed amount of microcrystalline cellulose, crospovidone and magnesium stearate, and then compressed into tablets.
Embodiment 3
[0035]
[0036] Preparation:
[0037] Mix the prescribed amount of amlodipine besylate and copovidone S630 evenly, heat under stirring at 62°C until the copovidone S630 melts, cool to 25°C, and solidify for 10 hours to obtain a drug-containing mixture; After a 60-mesh sieve, mix evenly with the prescribed amount of microcrystalline cellulose, crospovidone, and magnesium stearate, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com