Bendroflumethiazide tablet and preparation method thereof
Benfluflumethiazide tablets and Benfluflumethiazide technology, which are applied in the field of Benfluflumethiazide tablets and their preparation, can solve problems such as content differences among bendrofluthiazide tablets, and achieve easy industrialized large-scale production and preparation processes Simple, fast-dissolving results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
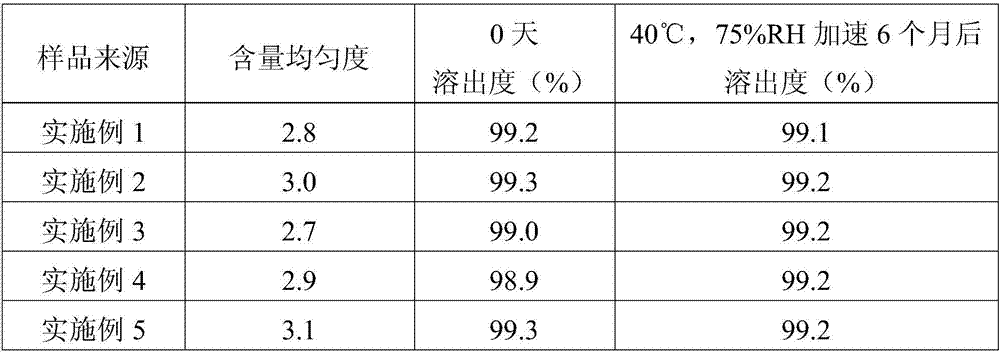
[0026] This embodiment provides a bendroflumethiazide tablet, which is made up of the following components in parts by weight: bendroflumethiazide 0.25Kg, microcrystalline cellulose PH-102 4Kg, lactose 6Kg, carboxymethyl starch sodium 0.4Kg, hard Magnesium fatty acid 0.03Kg.
[0027] The preparation method of above-mentioned bendroflumethiazide tablet, comprises the following steps:
[0028] (1) Perform jet milling of bendroflumethiazide, and the jet milling control D90=30-60 microns;
[0029] (2) get bendroflumethiazide, microcrystalline cellulose PH-102 and magnesium stearate and mix uniformly to obtain a mixed material;
[0030] (3) Add lactose and carboxymethyl starch sodium into the mixture, and mix well;
[0031] (4) Direct tablet compression by dry method, with a batch size of 100,000 tablets and a tablet specification of 2.5 mg / tablet.
Embodiment 2
[0033] This embodiment provides a bendroflumethiazide tablet, which is composed of the following components in parts by weight: bendroflumethiazide 0.25Kg, microcrystalline cellulose PH-102 6Kg, lactose 4Kg, carboxymethyl starch sodium 0.6Kg, hard Magnesium fatty acid 0.02Kg.
[0034] The preparation method of above-mentioned bendroflumethiazide tablet, comprises the following steps:
[0035] (1) Perform jet milling of bendroflumethiazide, and the jet milling control D90=30-60 microns;
[0036] (2) get bendroflumethiazide, microcrystalline cellulose PH-102 and magnesium stearate and mix uniformly to obtain a mixed material;
[0037] (3) Add lactose and carboxymethyl starch sodium into the mixture, and mix well;
[0038] (4) Direct tablet compression by dry method, with a batch size of 100,000 tablets and a tablet specification of 2.5 mg / tablet.
Embodiment 3
[0040] This embodiment provides a bendroflumethiazide tablet, which is composed of the following components in parts by weight: bendroflumethiazide 0.25Kg, microcrystalline cellulose PH-102 2Kg, lactose 8Kg, carboxymethyl starch sodium 0.2Kg, hard Magnesium fatty acid 0.04Kg.
[0041] The preparation method of above-mentioned bendroflumethiazide tablet, comprises the following steps:
[0042] (1) Perform jet milling of bendroflumethiazide, and the jet milling control D90=30-60 microns;
[0043] (2) get bendroflumethiazide, microcrystalline cellulose PH-102 and magnesium stearate and mix uniformly to obtain a mixed material;
[0044] (3) Add lactose and carboxymethyl starch sodium into the mixture, and mix well;
[0045] (4) Direct tablet compression by dry method, with a batch size of 100,000 tablets and a tablet specification of 2.5 mg / tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com