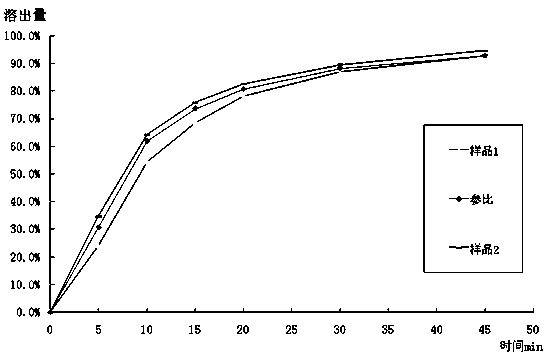
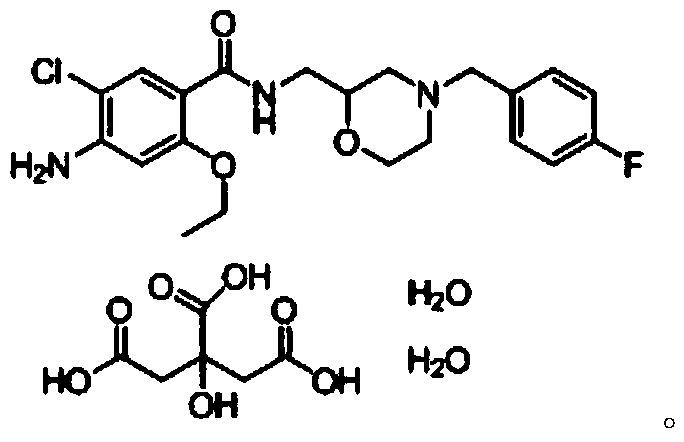
Mosapride citrate tablet and preparation method thereof
A technology of mosapride citrate and magnesium stearate, which is applied in the field of medical preparations containing organic active ingredients, can solve problems such as difficulty in investigating the optimal process, and achieves the advantages of being beneficial to industrialized production and having good medicinal properties and stability. The effect of the universality and the universality of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Mosapride Citrate 26.50g
[0040] Lactose 295.00g
[0041] Starch 160.00g
[0042] Low-substituted hydroxypropyl cellulose 140.00g
[0043] Hypromellose 12.00g
[0044] Purified water 188.00g
[0045] Magnesium Stearate 6.50g
[0046] Micronized silica gel 8.25g.
[0047] Preparation:
[0048] 1) Take raw and auxiliary materials respectively according to the above-mentioned prescription quantity, and the auxiliary materials are passed through a 60-mesh sieve;
[0049] 2) Weigh the purified water of the prescribed amount, add the hypromellose of the prescribed amount, stir and dissolve, and stir and dissolve to form the hypromellose aqueous solution;
[0050]3) Transfer lactose, API, starch and the hypromellose aqueous solution formed in step (2) to a wet granulator in sequence, and wet granulate after premixing;
[0051] 4) After drying, pass through a 30-mesh sieve for sizing and blend low-substituted hydroxypropyl cellulose, magnesium stearate and micropowder ...
Embodiment 2
[0054] Mosapride Citrate 26.50g
[0055] Lactose 300.00g
[0056] Starch 150.00g
[0057] Low-substituted hydroxypropyl cellulose 155.00g
[0058] Hypromellose 15.00g
[0059] Purified water 188.00g
[0060] Magnesium Stearate 6.50g
[0061] Micronized silica gel 8.00g.
[0062] Preparation:
[0063] 1) Take raw and auxiliary materials respectively according to the above-mentioned prescription quantity, and the auxiliary materials are passed through a 60-mesh sieve;
[0064] 2) Weigh the purified water of the prescribed amount, add the hypromellose of the prescribed amount, stir and dissolve to form the hypromellose aqueous solution;
[0065] 3) Transfer lactose, API, starch and the hypromellose aqueous solution formed in step (2) to a wet granulator in sequence, and wet granulate after premixing;
[0066] 4) After drying, pass through a 30-mesh sieve for sizing and blend low-substituted hydroxypropyl cellulose, magnesium stearate and micropowder silica gel; 5) Tablet ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com