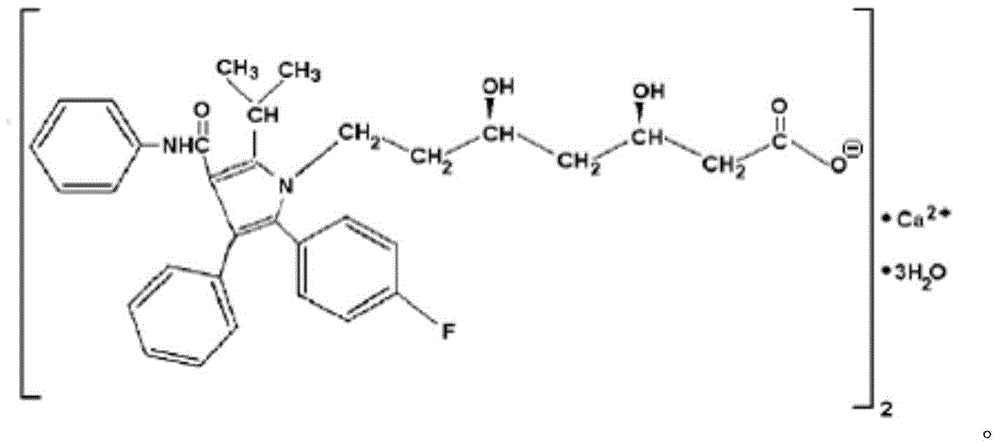
A kind of atorvastatin calcium tablet and preparation method thereof
A technology of atorvastatin calcium and tablet is applied in the field of tablet containing atorvastatin calcium and its preparation field, which can solve the problem of insufficient dissolution rate, surfactant gastrointestinal irritation, aggravating gastrointestinal irritation, etc. problem, to solve the stability problem, improve the solubility of the drug, and reduce the effect of stomach discomfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Atorvastatin calcium coated pellets
[0038]
[0039] (2) Atorvastatin Calcium Tablets
[0040]
[0041] Preparation Process:
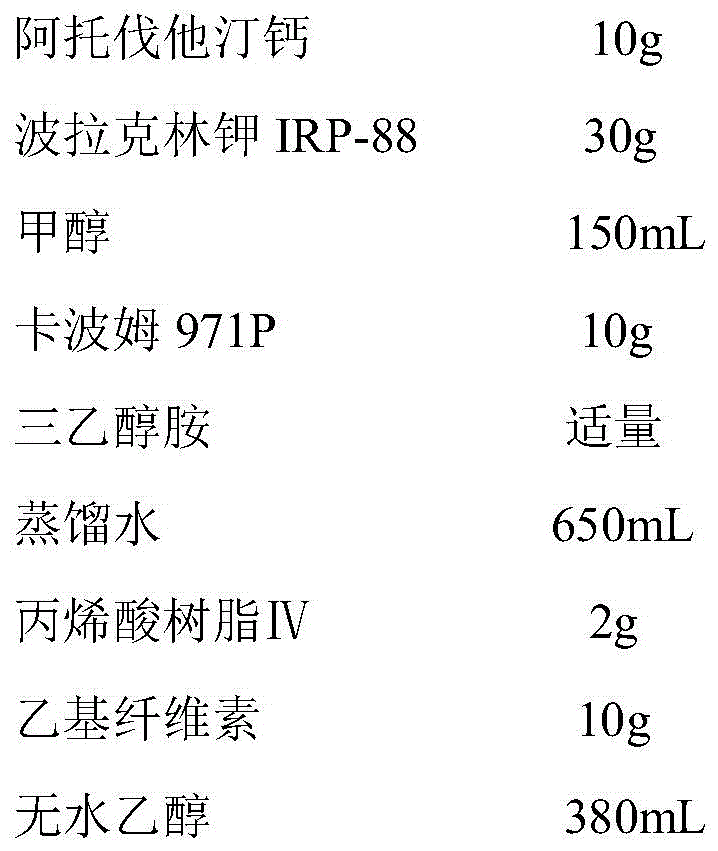
[0042] (1) Dissolve atorvastatin calcium in methanol, add polacrilin potassium IRP-88 fine powder, ultrasonicate for 1h, frequency 26.4kHz, dry under reduced pressure at 50°C to remove solvent, pulverize, and pass through a 100-mesh sieve to obtain atorvastatin calcium Vastatin calcium solid dispersion;
[0043] (2) Swell Carbomer 971P in water completely, add triethanolamine to adjust the pH to 9.0, add the atorvastatin calcium solid dispersion prepared in step (1) and stir evenly, and spray dry to obtain atorvastatin calcium pellets ;
[0044] (3) Dissolve acrylic resin IV and ethyl cellulose in absolute ethanol as a coating liquid, and carry out fluidized bed bottom spray coating to the atorvastatin calcium pellets prepared in step (3), with a blast flow rate of 80m 3 / h, the air inlet temperature is 60°C, and the weight gain is ...
Embodiment 2
[0047] (1) Atorvastatin calcium coated pellets
[0048]
[0049] (2) Atorvastatin Calcium Tablets
[0050]
[0051]
[0052] Preparation Process:
[0053] (1) Dissolve atorvastatin calcium in methanol, add polacrilin potassium IRP-88 fine powder, ultrasonicate for 1h, frequency 26.4kHz, dry under reduced pressure at 50°C to remove solvent, pulverize, and pass through a 100-mesh sieve to obtain atorvastatin calcium Vastatin calcium solid dispersion;
[0054] (2) Swell Carbomer 971P in water completely, add triethanolamine to adjust the pH to 10.0, add the atorvastatin calcium solid dispersion prepared in step (1) and stir evenly, and spray dry to obtain atorvastatin calcium pellets ;
[0055] (3) Dissolve acrylic resin IV and ethyl cellulose in absolute ethanol as a coating liquid, and carry out fluidized bed bottom spray coating to the atorvastatin calcium pellets prepared in step (3), with a blast flow rate of 80m 3 / h, the air inlet temperature is 60°C, and the ...
Embodiment 3
[0058] (1) Atorvastatin calcium coated pellets
[0059]
[0060] (2) Atorvastatin Calcium Tablets
[0061]
[0062] Preparation Process:
[0063] (1) Dissolve atorvastatin calcium in methanol, add polacrilin potassium IRP-88 fine powder, ultrasonicate for 1h, frequency 26.4kHz, dry under reduced pressure at 50°C to remove solvent, pulverize, pass through a 100-mesh sieve to obtain atorvastatin Statin calcium solid dispersion;
[0064] (2) Swell Carbomer 971P in water completely, add triethanolamine to adjust the pH to 11.0, add the atorvastatin calcium solid dispersion prepared in step (1) and stir evenly, and spray dry to obtain atorvastatin calcium pellets ;
[0065] (3) Dissolve acrylic resin IV and ethyl cellulose in absolute ethanol as a coating liquid, and carry out fluidized bed bottom spray coating to the atorvastatin calcium pellets prepared in step (3), with a blast flow rate of 80m 3 / h, the air inlet temperature is 60°C, and the weight gain is 20%, to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com