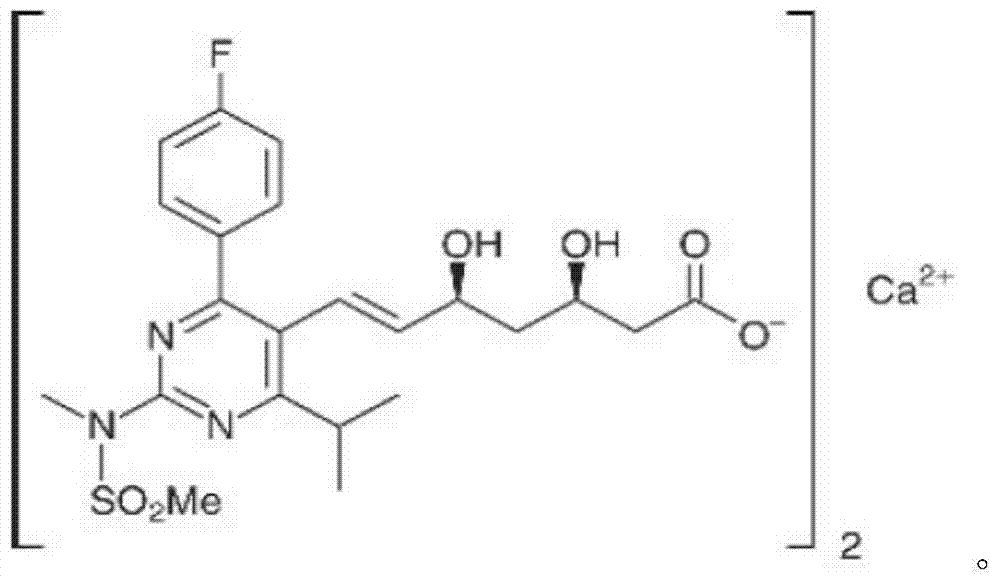
Rosuvastatin calcium tablet and preparation process thereof
A technology for rosuvastatin calcium and a preparation process is applied in the field of rosuvastatin calcium-containing tablets and the preparation process thereof, and can solve the problems of stable rosuvastatin calcium tablets, high fluidity requirements, and poor particle size. Uniformity and other problems, to achieve the effect of easy industrialized large-scale production, solving the release stability, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Preparation process: The prescription quantity weighed rosuvastatin calcium, polystyrene maleic anhydride copolymer, and poloxamer 407 through a 100-mesh sieve, added to absolute ethanol, suspended, and dried under reduced pressure at 40°C to remove ethanol. The dry matter is passed through a 80-mesh sieve, mixed evenly with the prescribed amount of hydroxypropyl methylcellulose, sodium alginate, and microcrystalline cellulose passed through a 100-mesh sieve, then mixed with magnesium stearate, and compressed into tablets.
Embodiment 2
[0037]
[0038]
[0039] Preparation process: The prescription quantity weighed rosuvastatin calcium, polystyrene maleic anhydride copolymer, and poloxamer 407 through a 100-mesh sieve, added to absolute ethanol, suspended, and dried under reduced pressure at 40°C to remove ethanol. The dried product is passed through a 80-mesh sieve, mixed evenly with the prescribed amount of hydroxypropyl methylcellulose, sodium alginate, and starch passed through a 100-mesh sieve, then mixed with calcium stearate, and compressed into tablets.
Embodiment 3
[0041]
[0042] Preparation process: The prescription quantity weighed rosuvastatin calcium, polystyrene maleic anhydride copolymer, and poloxamer 407 through a 100-mesh sieve, added to absolute ethanol, suspended, and dried under reduced pressure at 40°C to remove ethanol. The dried product is passed through an 80-mesh sieve, mixed evenly with the prescribed amount of hydroxypropyl methylcellulose, sodium alginate, and mannitol passed through a 100-mesh sieve, then mixed with sodium stearate fumarate, and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com