Azilsartan intermediate and preparation method thereof
An intermediate and reaction technology, which is applied to the intermediate of synthesizing antihypertensive drug azilsartan and the field of preparation thereof, can solve the problems of many impurities, low process yield, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Preparation of compound (3B)
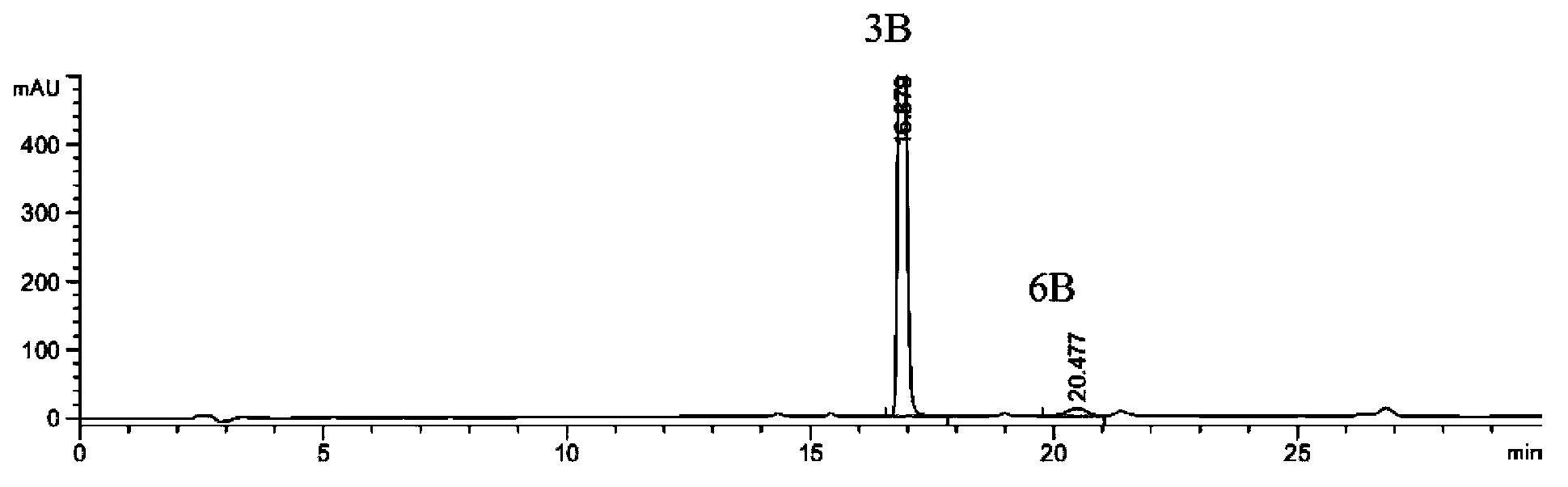
[0085] 15.2 g of the raw material (compound 2B) was placed in a reaction flask, 150 ml of ethanol, 3.6 g of triethylamine, and 28 ml of 50% aqueous hydroxylamine solution were added, reacted for 7 hours, cooled and crystallized to obtain 14.1 g (86.0%) of a white solid. The main amide impurity (compound 6B) in the reaction solution was detected by HPLC at the end of the reaction: the product was 2.55%: 97.44% (that is, the ratio of impurity to product was 1:38.2), see figure 1 .
[0086] Table 1: figure 1 Analysis of HPLC detection data
[0087]
[0088] The mass spectrum shows: the molecular ion peak [M+1] is 459.
[0089] 1 HNMR spectrum (DMSO-d6): δppm 1.1-1.3 (3H, t), 1.3-1.4 (3H, t), 3.2-3.3 (2H), 4.1-4.3 (2H, q), 4.5-4.7 (2H, q ), 5.4-5.5 (1H, s), 5.5-5.6 (2H, s), 6.9-7.8 (11H, m).
[0090] Melting point: 210-212°C.
[0091] Impurity 6B
[0092] Mass spectrum shows: molecular ion peak [M+1] is 444.
[0093] Melting point:...
Embodiment 2
[0095] Preparation of compound (3B)
[0096] 15.0 g of raw material (compound 2B) was placed in a reaction flask, 150 ml of ethanol, 1.8 g (2.5 mL) of triethylamine, and 30 ml of 50% aqueous hydroxylamine solution were added, reacted for 10 h, cooled and crystallized, and 15.4 g (95.2%) of a white solid was obtained. . The main amide impurity (compound 6B) in the reaction solution was detected by HPLC at the end of the reaction: the product was 2.55%:97.44% (that is, the ratio of impurities to product was 1:38.2), and the purity of the product detected by HPLC after cooling and crystallization was 98.49%.
Embodiment 3
[0105] Preparation of compound (3B)
[0106] 7.5 g of the raw material (compound 2B) was placed in a reaction flask, 50 ml of ethanol and 15 ml of 50% aqueous hydroxylamine solution were added, reacted for 10 h, cooled and crystallized to obtain 6.2 g (76.0%) of a white solid. The main amide impurity (compound 6B) in the reaction solution was detected by HPLC: the product was 12.16%: 87.83%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com