Preparation method of dabigatran etexilate mesylate
A technology for dabigatran etexilate mesylate and methanesulfonic acid, which is applied in the field of medicine and can solve the problems of short synthesis route, high cost, and unsuitability for industrial production and use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
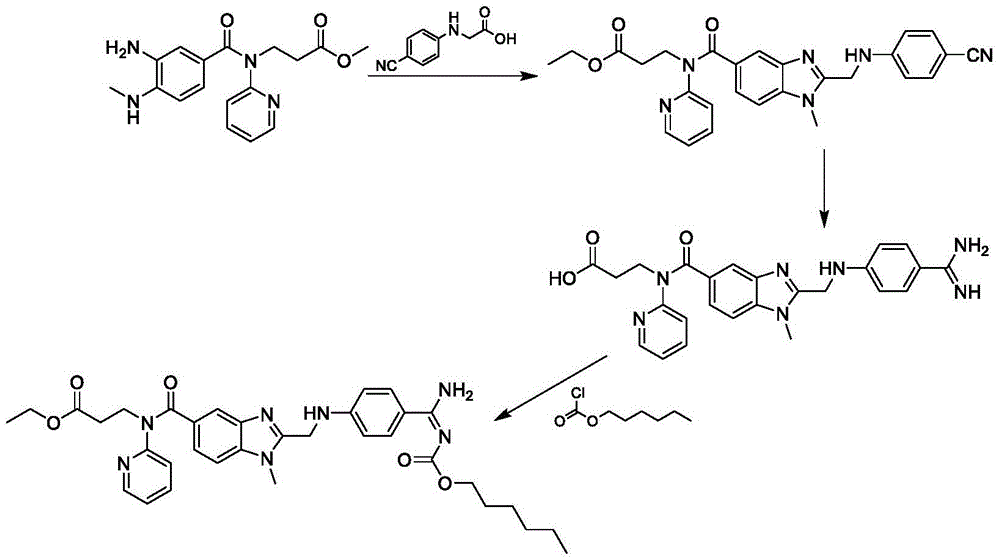
[0070] This embodiment provides a kind of preparation method of dabigatran etexilate mesylate, prepare according to the following steps:
[0071] 1. Preparation of intermediate S3
[0072] Take 0.38kg (2.3mol) of N,N'-carbonyldiimidazole and add it to 4.5L tetrahydrofuran, stir at room temperature and add 0.36kg (2mol) of N-(4-cyanophenyl)-aminoacetic acid, stir for 30 minutes, and then Add 0.62 kg (1.8 mol) of ethyl 3-[(3-amino-4-methylaminobenzoyl)pyridin-2-ylamino]propionate, and keep the reaction at room temperature for 10-12 hours. Add 0.05kg of purified water, stir for 10 minutes, concentrate under reduced pressure at 30-60°C, and dry in vacuo to obtain 0.85kg of intermediate (S3), with a yield of 93.8%.
[0073] 2. Preparation of intermediate S4
[0074] Add 0.80 kg (1.6 mol) of the intermediate (S3) obtained in step 1 to 2.5 kg of n-butyl acetate and 0.3 kg of glacial acetic acid, heat up to 85-90° C. and react for 3-4 hours. Subsequently, n-butyl acetate and glacia...
Embodiment 2
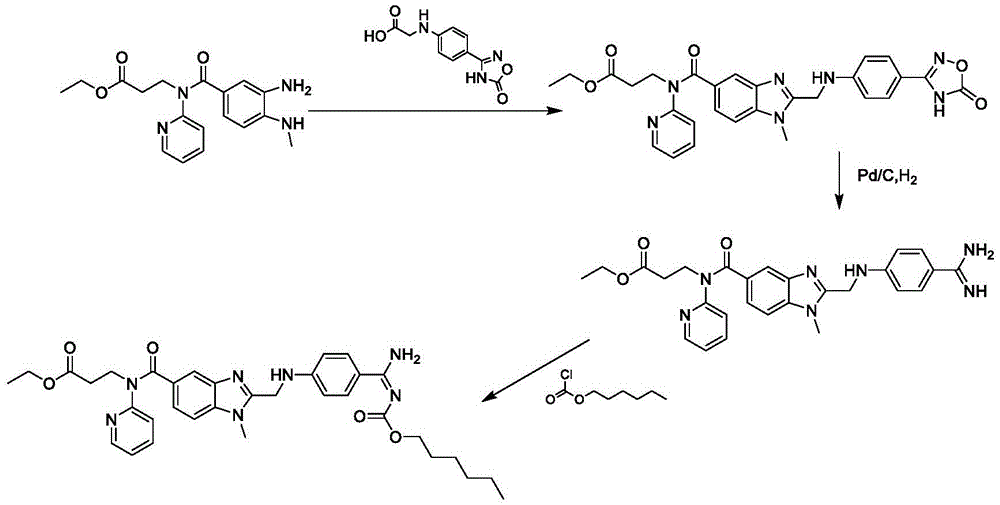
[0082] This embodiment provides a kind of preparation method of dabigatran etexilate mesylate, prepare according to the following steps:
[0083] 1. Preparation of intermediate S3
[0084] Take 0.38kg (2.3mol) of N,N'-carbonyldiimidazole and add it to 4.5L tetrahydrofuran, stir at room temperature and add 0.36kg (2mol) of N-(4-cyanophenyl)-aminoacetic acid, stir for 30 minutes, and then Add 0.62 kg (1.8 mol) of ethyl 3-[(3-amino-4-methylaminobenzoyl)pyridin-2-ylamino]propionate, and keep the reaction at room temperature for 10-12 hours. Add 0.05 kg of purified water, stir for 10 minutes, concentrate under reduced pressure at 30-60° C., and dry in vacuo to obtain 0.83 kg of intermediate (S3), with a yield of 91.6%.
[0085] 2. Preparation of intermediate S4
[0086] Add 0.80 kg (1.6 mol) of the intermediate (S3) obtained in step 1 to 2.5 kg of n-butyl acetate and 0.3 kg of glacial acetic acid, heat up to 85-90° C. and react for 3-4 hours. Subsequently, n-butyl acetate and gl...
Embodiment 3
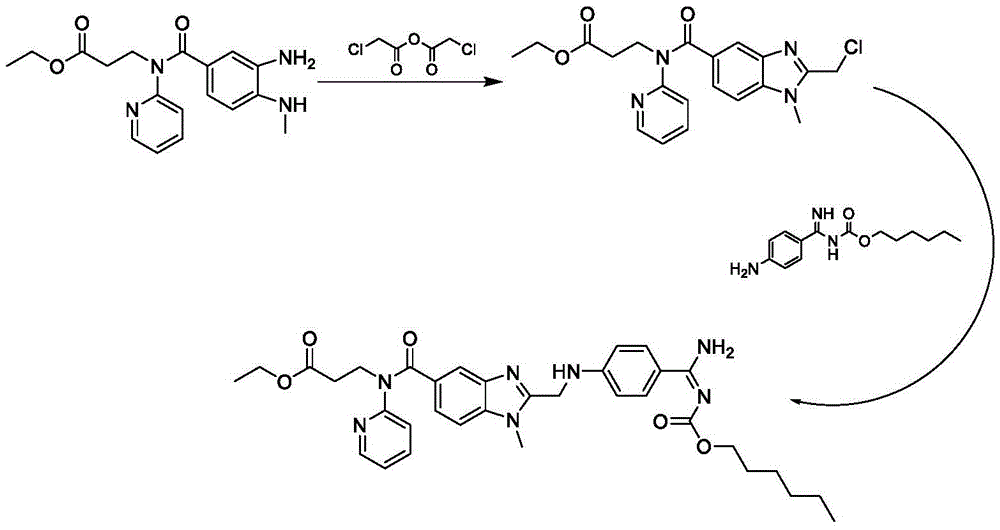
[0094] This embodiment provides a kind of preparation method of dabigatran etexilate mesylate, prepare according to the following steps:
[0095] 1. Preparation of intermediate S3
[0096] Take 0.47kg (2.3mol) of dicyclohexylcarbodiimide and add it to 4.5L tetrahydrofuran, stir and add 0.36kg (2mol) of N-(4-cyanophenyl)-aminoacetic acid at room temperature, stir for 30 minutes, and then add 0.62 kg (1.8 mol) of ethyl 3-[(3-amino-4-methylaminobenzoyl)pyridin-2-ylamino]propionate was kept at room temperature for 10-12 hours. Add 0.05kg of purified water, stir for 10 minutes, concentrate under reduced pressure at 30-60°C, and dry in vacuo to obtain 0.81kg of intermediate (S3), with a yield of 89.4%.
[0097] 2. Preparation of intermediate S4
[0098] Add 0.75 kg (1.5 mol) of the intermediate (S3) obtained in Step 1 to 2.5 kg of ethyl acetate and 0.3 kg of glacial acetic acid, and heat up to reflux for 5-6 hours. Ethyl acetate and glacial acetic acid were then distilled off und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com