Method for carrying out carbalkoxylation acylation on fluorouracil compound with active coupling agent
A technology of alkoxycarbonylation and alkoxycarbonyl, which is applied in the application field of alkoxycarbonylation of fluorinated pyrimidine compounds and the synthesis of capecitabine, which can solve the problems of unfavorable industrial production and achieve easy control and process Simple process and equipment, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
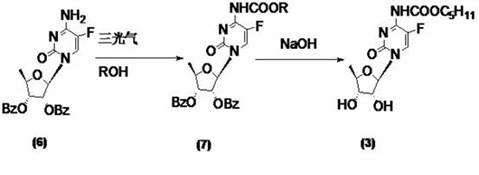
Image
Examples
Embodiment 1
[0058]
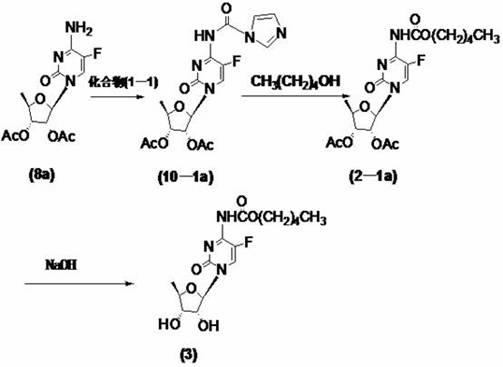
[0059] At room temperature, dissolve 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (8a) (10g, 30mmol) with 400ml of dichloromethane and add 1,1' -Carbonyldiimidazole (1-1) (9.7g, 60mmol), TLC until the reaction is complete (developing solvent: dichloromethane / methanol=12:1), add n-pentanol (6.6ml, 60mmol), TLC until the reaction is complete (Developer: dichloromethane / methanol=12:1), washed with 50ml×3 water, dried the organic phase with anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness to obtain a yellow oil, namely compound (2-1a) 10.7 g, yield 80.5%.
Embodiment 2
[0060] Example 2: Preparation of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]-cytidine nucleoside (2-1a).
[0061] At room temperature, dissolve 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (8a) (10g, 30mmol) with 400ml of dichloromethane and add 1,1' -Carbonyldiimidazole (1-1) (13.2g, 80mmol), TLC until the reaction is complete (developing solvent: dichloromethane / methanol=12:1), add n-pentanol (8.7ml, 80mmol), TLC until the reaction is complete (Developer: dichloromethane / methanol=12:1), washed with 50ml×3 water, dried the organic phase with anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness to obtain a yellow oil, namely compound (2-1a) 10.8 g, yield 81.5%.
Embodiment 3
[0062] Example 3: Preparation of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]-cytidine nucleoside (2-1a).
[0063] At room temperature, the compound of formula (8a) (10g, 30mmol) was stirred and dissolved in 400ml of dichloromethane, and N,N'-disuccinimidyl carbonate (DSC) (15.4g, 60mmol) was added, TLC until the reaction was complete (Developer: dichloromethane / methanol=12:1), filter off the white solid, add n-pentanol (6.6ml, 60mmol) to the filtrate, TLC until the reaction is complete (developer: dichloromethane / methanol=12: 1), washed with 50ml×3 water, dried the organic phase with anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness to obtain 9.2g of compound (2-1a) as a yellow oil, with a yield of 68.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com