Preparation method of 2'3'-di-O-acetyl-5'-desoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine
A technology of pentoxycarbonyl and acetyl groups, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of difficult removal of pyridine, high residue of pyridine, difficult treatment of waste water, etc., and achieve good product quality, The effect of less environmental hazard and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
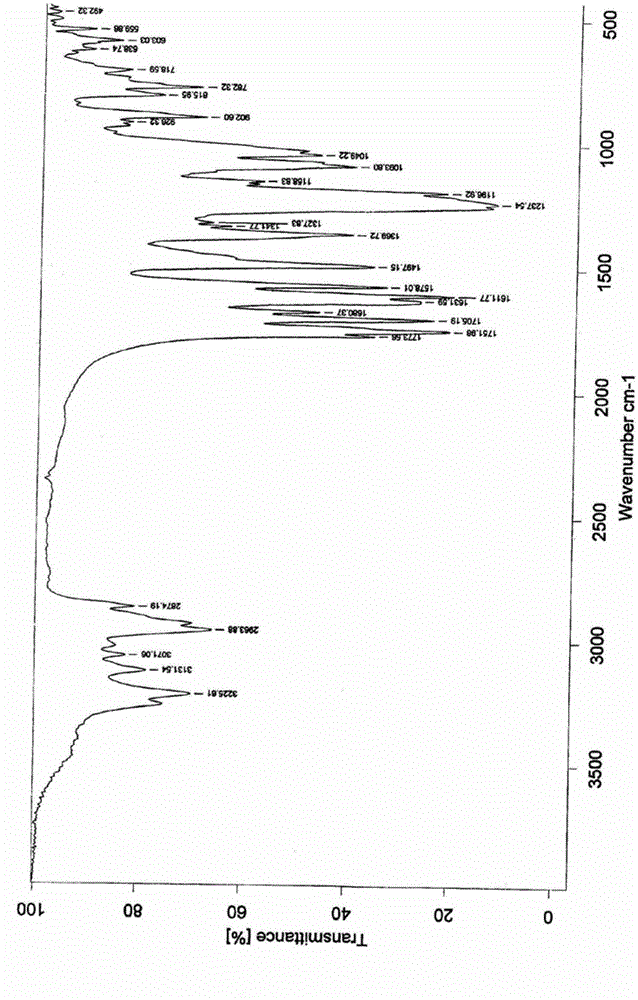
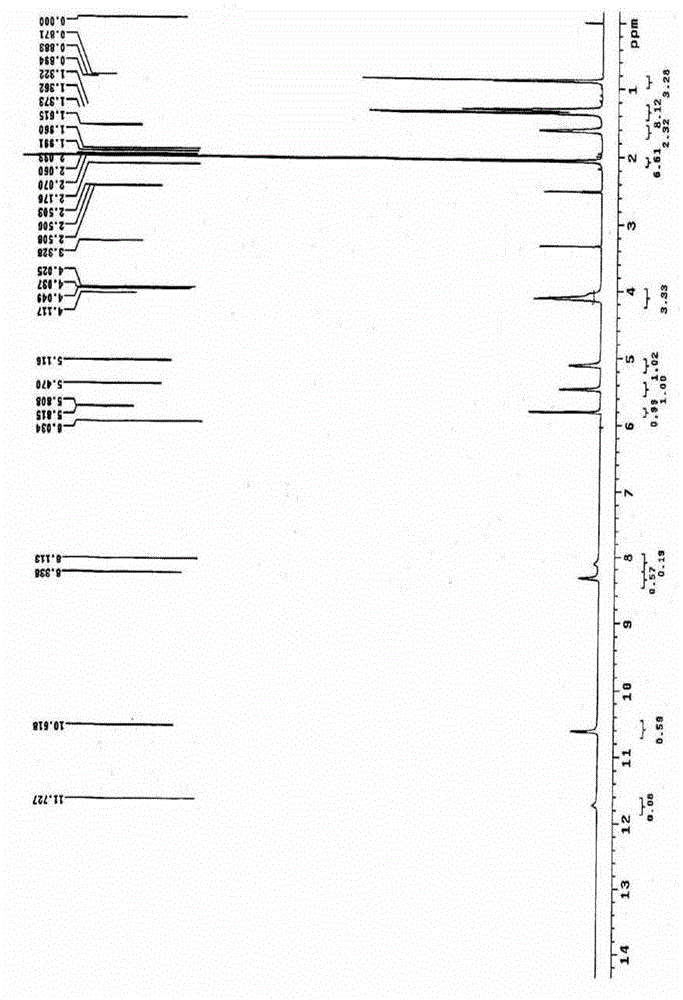
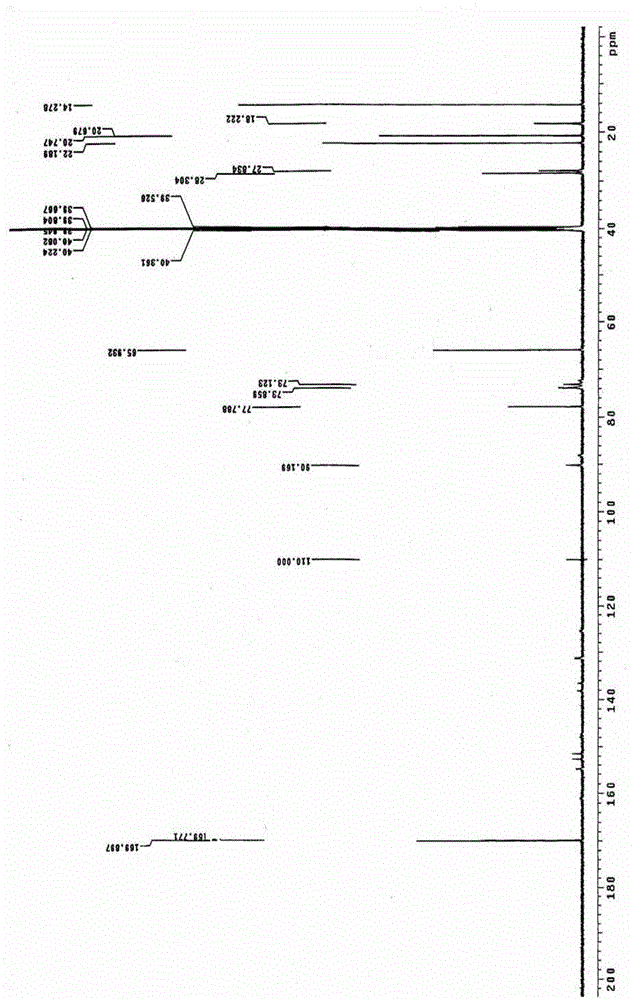
Image
Examples
Embodiment 1
[0027] Put 32.9g (0.1mol) of 2'3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, 380ml of dichloromethane, and 63.6g of anhydrous sodium carbonate into a 1000ml three-necked flask. ), 0.24g (2mmol) of 4-dimethylaminopyridine, 5ml (2mmol) of 10% tetra-n-butylammonium hydroxide, 42.5ml (0.3mol) of n-pentyl chloroformate was added dropwise at room temperature, after addition, the temperature was raised to reflux for 5h, Cool down to room temperature, filter the reaction system with suction, add 70ml of purified water to the filtrate, stir, let stand for stratification, discard the water layer, stir the organic phase with 70ml of saturated sodium bicarbonate solution, let stand for stratification, discard the water layer, do not use directly proceed to the next reaction; or continue to add 30g of anhydrous sodium sulfate to the organic phase to dry, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize with ether to obtain a white solid 2'3'-di-O-acetyl -5'...
Embodiment 2
[0029] Put 32.9g (0.1mol) of 2'3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, 380ml of dichloromethane, and 82.8g of anhydrous potassium carbonate into a 1000ml three-necked flask. ), 0.24g (2mmol) of 4-dimethylaminopyridine, 5ml (2mmol) of 10% tetra-n-butylammonium hydroxide, 42.5ml (0.3mol) of n-pentyl chloroformate was added dropwise at room temperature, after the addition was completed, the temperature was raised to reflux for 9h, Cool down to room temperature, filter the reaction system with suction, add 70ml of purified water to the filtrate, stir, let stand for stratification, discard the water layer, stir the organic phase with 70ml of saturated sodium bicarbonate solution, let stand for stratification, discard the water layer, do not use directly proceed to the next reaction; or continue to add 30g of anhydrous sodium sulfate to the organic phase to dry, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize with ether to obtain a white solid ...
Embodiment 3
[0031] Put 32.9g (0.1mol) of 2'3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, 380ml of dichloromethane, and 63.6g of anhydrous sodium carbonate into a 1000ml three-necked flask. ), 0.24g (2mmol) of 4-dimethylaminopyridine, 10ml (4mmol) of 10% tetra-n-butylammonium hydroxide, 42.5ml (0.3mol) of n-pentyl chloroformate was added dropwise at room temperature, after the addition was completed, the temperature was raised to reflux for 5h, Cool down to room temperature, filter the reaction system with suction, add 70ml of purified water to the filtrate, stir, let stand for stratification, discard the water layer, stir the organic phase with 70ml of saturated sodium bicarbonate solution, let stand for stratification, discard the water layer, do not use directly proceed to the next reaction; or continue to add 30g of anhydrous sodium sulfate to the organic phase to dry, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize with ether to obtain a white solid 2'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com