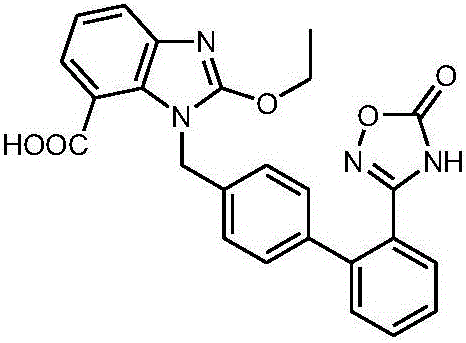
Azilsartan pellet tablet and preparation method thereof
A technology of azilsartan pellets and pellets, which is applied in the fields of pill delivery, microcapsules, cardiovascular system diseases, etc. It can solve the problems of organic solvent residue, high control requirements, difficult process operation, etc., and increase the surface area , Gastric emptying effect is small, and the effect of reducing high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
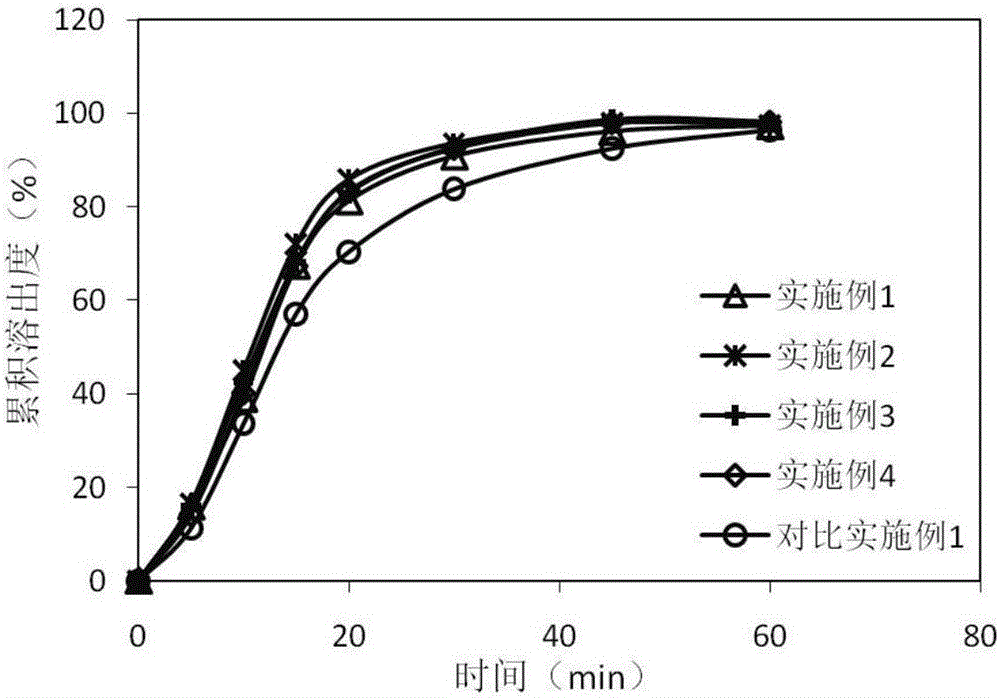
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of azilsartan pellets.
[0029] (1) Preparation of Azilsartan pellets:
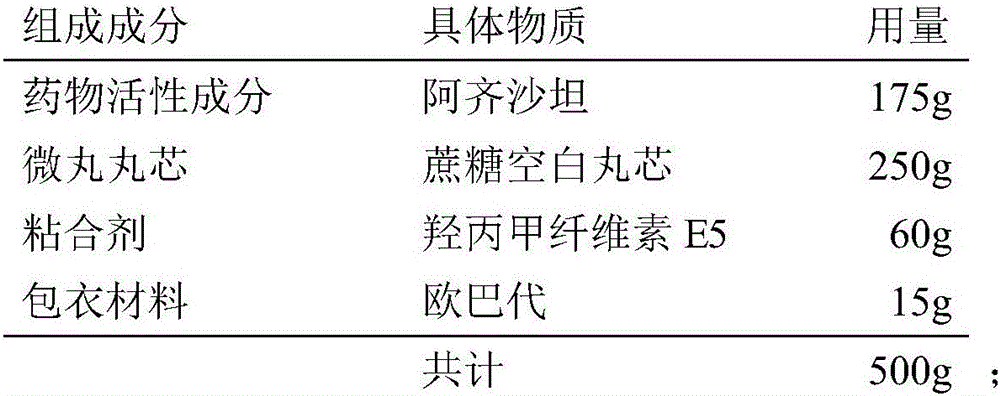
[0030] Weigh the components according to the following formula:
[0031]
[0032] Dissolve hypromellose E5 in 60% ethanol solution to obtain a 3.5% (w / v) adhesive solution, then add azilsartan to the adhesive solution, and after dispersing evenly, obtain the drug Coating liquid; put the sucrose blank core in the fluidized bed, start the bottom spray program, preheat, and after the temperature of the material reaches equilibrium, spray the coating liquid with the drug to obtain the core containing the drug; dissolve Opadry in In water, an 8% (w / v) coating solution was obtained, which was sprayed into a fluidized bed for film coating to obtain azilsartan pellets.
[0033] (2) Preparation of Azilsartan Micropellets:
[0034] Weigh the components according to the following formula:
[0035]
[0036] Add lactose, pregelatinized starch, hydroxypropyl cellulose, sod...
Embodiment 2
[0037] Embodiment 2: Preparation of azilsartan pellets.
[0038] (1) Preparation of Azilsartan pellets:
[0039] Weigh the components according to the following formula:
[0040]
[0041] Dissolve povidone K30 in 60% ethanol solution to obtain a 3.5% (w / v) adhesive solution, then add azilsartan to the adhesive solution, and disperse evenly to obtain a drug-coated coating solution; put the sucrose blank ball core in the fluidized bed, start the bottom spray program, preheat, and after the temperature of the material reaches equilibrium, spray into the coating solution to obtain the drug-containing ball core; dissolve Opadry in water, An 8% (w / v) coating solution was obtained, which was sprayed into a fluidized bed for film coating to obtain azilsartan pellets.
[0042] (2) Preparation of Azilsartan Micropellets:
[0043] Weigh the components according to the following formula:
[0044]
[0045]
[0046] Add microcrystalline cellulose, hydroxypropyl cellulose, low-s...
Embodiment 3
[0047] Embodiment 3: Preparation of azilsartan pellets.
[0048] (1) Preparation of Azilsartan pellets:
[0049] Weigh the components according to the following formula:
[0050]
[0051] Dissolve hydroxypropyl cellulose in 60% ethanol solution to obtain a 3.5% (w / v) binder solution, then add azilsartan to the binder solution, and after dispersing evenly, obtain a drug pack Coating liquid; put the microcrystalline cellulose blank pellet core in the fluidized bed, start the bottom spray program, preheat, and after the temperature of the material reaches equilibrium, spray the coating solution with the drug to obtain the pellet core containing the drug; put Opadry It is dissolved in water to obtain an 8% (w / v) coating solution, which is sprayed into a fluidized bed for film coating to obtain azilsartan pellets.
[0052] (2) Preparation of Azilsartan Micropellets:
[0053] Weigh the components according to the following formula:
[0054]
[0055] Add lactose, microcrystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com