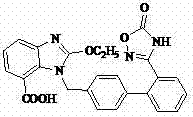
Azilsartan polymorph and preparation method thereof
A technology of polymorph and crystal form, applied in the direction of organic chemistry, can solve the problems of difficult removal of impurities, unsatisfactory removal effect, poor solubility, etc., and achieve the effect of easy separation, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
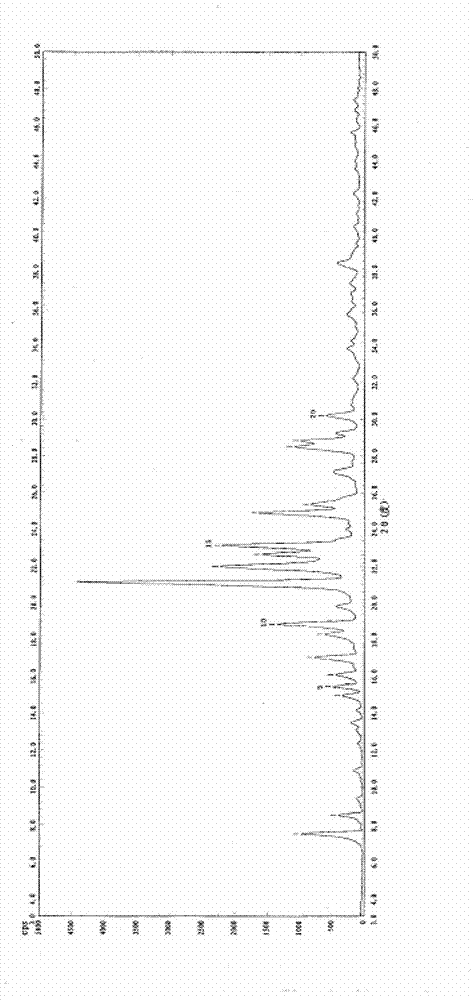
[0019] Example 1: Recrystallization of azilsartan in ethanol-tetrahydrofuran system.
[0020] Add 0.3g of Azilsartan to the reaction flask, add 1.5mL of ethanol, raise the temperature to reflux, drop 1mL of tetrahydrofuran into the reaction flask, all the solids are dissolved, at the same temperature, after 2min, a white solid precipitates out, keep at the same temperature for 30min, and then cool down to room temperature (20°C), filtered, and the obtained solid was air-dried at 60°C to constant weight to obtain the crystal form B of azilsartan. Purity>99%, simple impurity<0.1%.
[0021]
example 2
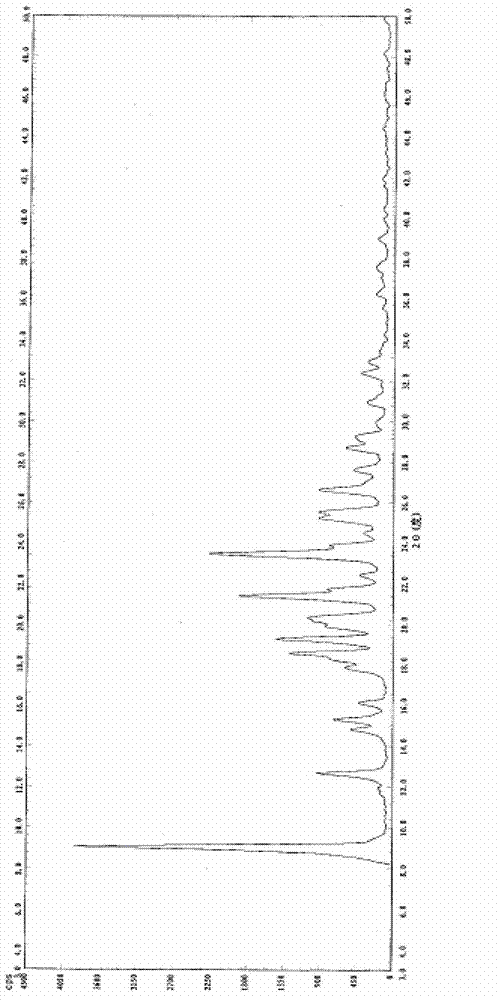
[0022] Example 2: Recrystallization of azilsartan in water-tetrahydrofuran system.
[0023] Add 42.6g of Azilsartan into the reaction flask, add 213mL of water, stir and raise the temperature to 70°C, slowly add 213mL of tetrahydrofuran into the reaction flask, the solid is completely dissolved, at the same temperature, after 30min, a white solid gradually precipitates out, continue to be at the same temperature for 30min , lowered to room temperature (20°C), stirred for 1 h, filtered, and the obtained white solid was air-dried at 60°C to constant weight to obtain the crystal form B of azilsartan. The yield is 95.1%, the purity is >99%, and the single heterogeneity is <0.1%.
example 3
[0024] Example 3: Recrystallization of azilsartan in water-tetrahydrofuran system.
[0025] Add 7g of azilsartan to the reaction flask, add 35mL of water, add 35mL of tetrahydrofuran, heat up to reflux, 10min later, a white solid precipitates, after 30min, naturally cool to room temperature (20°C), filter, and the obtained solid is air-dried at 60°C to Constant weight, to obtain azilsartan crystal form B. Yield 97%, purity>99%.
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com