Azilsartan tablet and preparation method thereof
A technology for azilsartan tablets and tablets, applied in the field of azilsartan tablets and their preparation, can solve the problems of slow dissolution rate, incomplete dissolution, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2 and comparative example 1-8
[0030] The preparation of embodiment 1-2 and comparative example 1-8 azilsartan sheet
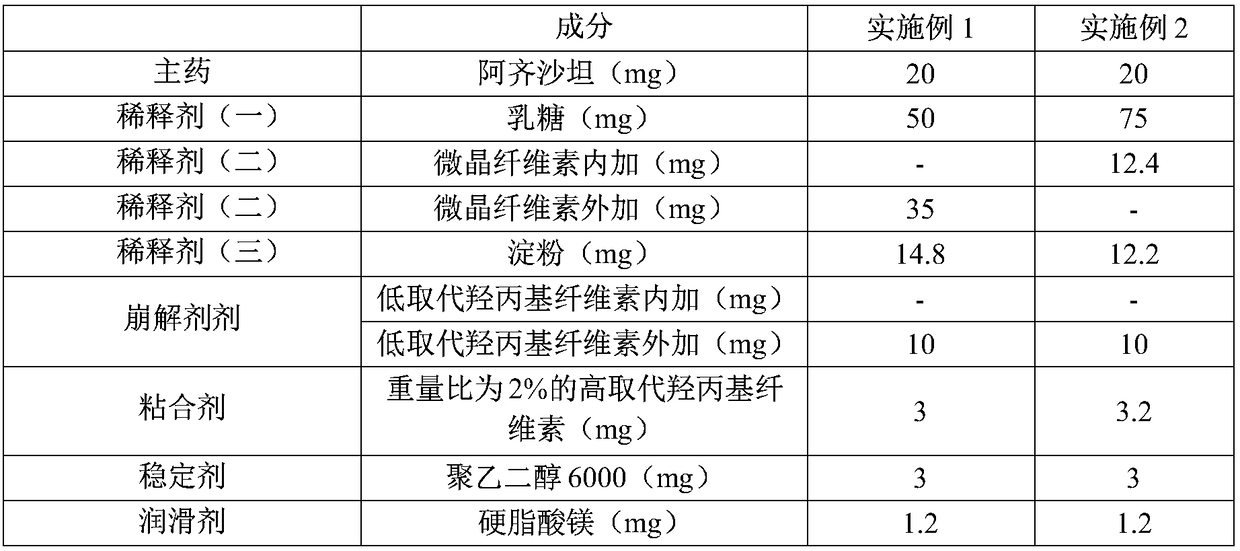
[0031] The composition of embodiment 1-2 azilsartan tablet is shown in the following table 1:
[0032] The azilsartan tablet composition of table 1 embodiment 1 and embodiment 2 (the tablet weight of described azilsartan tablet is 137mg)
[0033]
Embodiment 1
[0037] The preparation of embodiment 1 azilsartan tablet:
[0038] S1 Micronize azilsartan to control particle size D90 10-24 μm to obtain azilsartan micropowder;
[0039] S2 Weigh an appropriate amount of purified water, put it in a stainless steel bucket, add the binder under the condition of stirring, stir until dissolved, and prepare a solution with a concentration of 6-10%, then add the stabilizer under the condition of stirring and stir until Dissolved to obtain B solution;
[0040] S3 sequentially put diluent (2), diluent (1), azilsartan micropowder obtained in step S1, and diluent (3) into a wet mixing granulator, stir and shear to mix to obtain a mixture;
[0041] S4 Turn on the stirring paddle, spray the mixture obtained in step S3 evenly into the B solution obtained in step S2, stir for 60-150 s, cut for 50-120 s, granulate, granulate, and obtain wet granules;
[0042] S5 Pour the wet granules obtained in step S4 into the fluidized bed spray drying granulator, set: ...
Embodiment 2
[0044] Example 2 The preparation of Azilsartan Tablets refers to that shown in Example 1. Compared
[0045] Examples 1-8 The preparation of azilsartan tablets is shown in Example 1. Test case
[0046] 1. Quality inspection of Azilsartan tablets
[0047] The azilsartan tablets prepared in Examples 1-2 and Comparative Examples 1-8 were tested for angle of repose, hardness, disintegration time limit, and in vitro dissolution rate.
[0048] Tablet inspection items and testing methods:
[0049] ①Measurement of angle of repose Fix the iron ring on the hoop (the height is enough to allow the funnel to hang directly above the watch glass), place the watch glass right below the funnel, adjust the watch glass so that the origin is perpendicular to the funnel, Slowly add the material from the funnel until the edge of the watch glass can no longer contain the material, that is, until a regular cone is formed. Use a ruler to measure the height h of the material, and then measure the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com