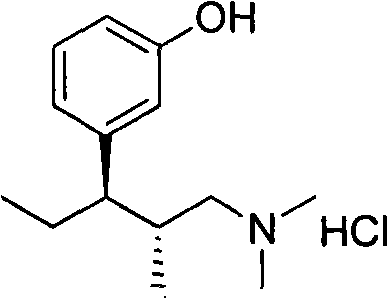
Method for preparing tapentadolhydrochloride and intermediate thereof
A technology of racemate and enantiomer, applied in the field of medicinal chemistry, to achieve the effect of sufficient market supply and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057]
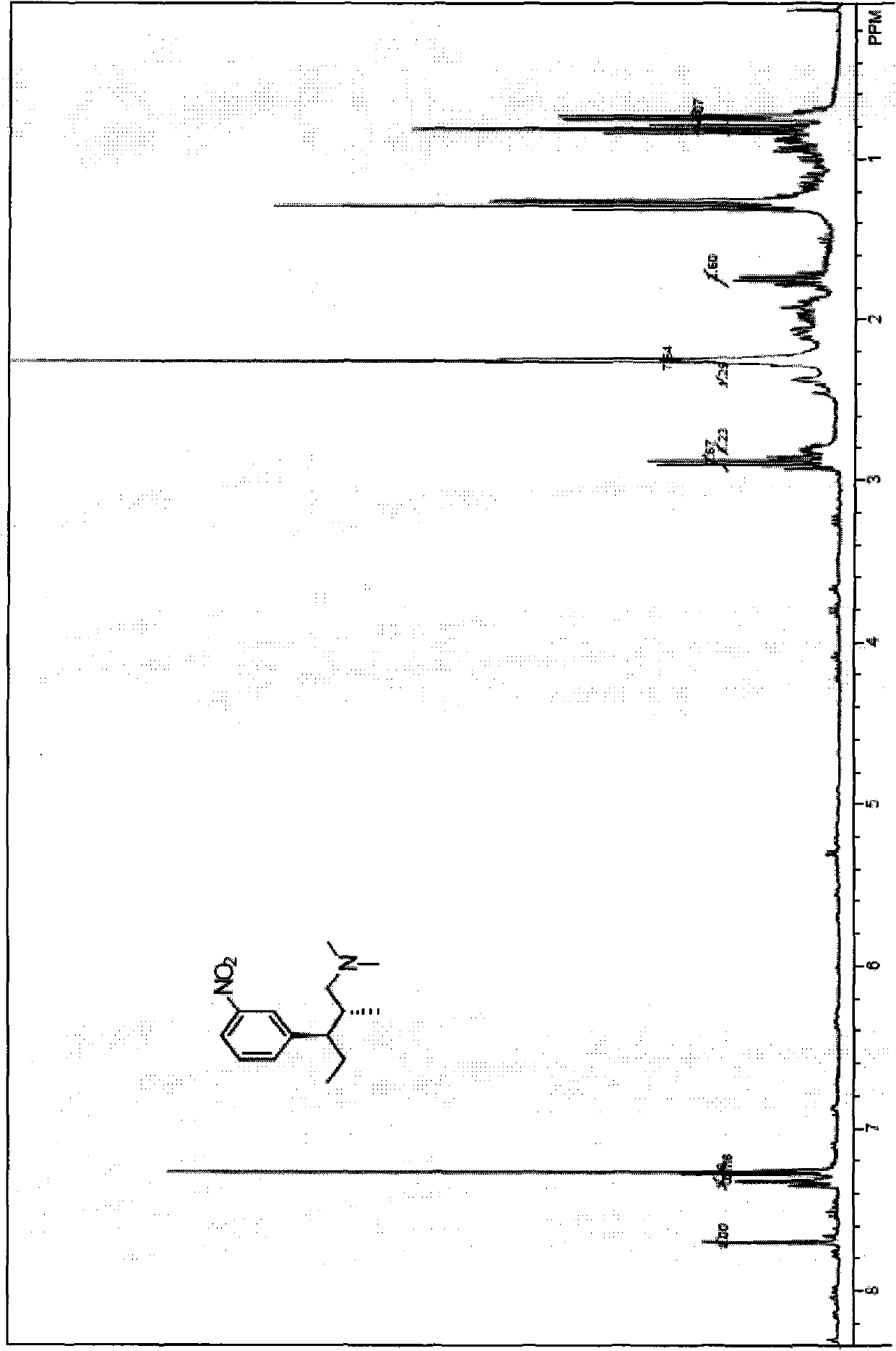
[0058] Put 30ml N,N-dimethylformamide, 3-tert-butyldimethylsiloxybenzaldehyde 14.2g (0.06mol, 1eq), and R-proline 2.07g (0.018mol) into a 100ml single-necked flask. , 0.3eq), slowly drip 8.8ml (0.12mol, 2eq) of propionaldehyde in an ice-water bath. After reacting for about 3h, add 30ml of water to stop the reaction; then add appropriate amount of ethyl acetate for extraction, dry, and spin to dry to obtain an oil (2R,3S)-3-hydroxy-2-methyl-3-(3-tert-butyldimethylsiloxyphenyl)propanal, the oily substance is directly put into the next reaction.
Embodiment 2
[0060]
[0061] Put 20ml of dichloromethane and 3.59g (0.044mol, 1.1eq) of dimethylamine hydrochloride into a 250ml single-necked flask. Slowly add 4.45g (0.044mol, 1.1eq) of triethylamine under ice water bath, and react for a while Then add the above-mentioned preparation oil (2R, 3S)-3-hydroxy-2-methyl-3-(3-tert-butyldimethylsiloxyphenyl)propanal (0.04mol, 1.0eq) in methanol 50ml, add NaBH4 in batches, and react at low temperature for 2h; then, add appropriate amount of water and ethyl acetate for extraction, dry, and spin dry to obtain an oily (1S, 2S)-3-(dimethylamino)-2 -Methyl-1-(3-tert-butyldimethylsiloxyphenyl)-1-propanol, the oily substance is directly put into the next reaction.
Embodiment 3
[0063]
[0064] In an ice-water bath, add the oily (1S, 2S)-3-(dimethylamino)-2-methyl-1-(3-tert-butyldimethylsiloxybenzene) prepared in Example 2 above. Yl)-1-propanol, put 50ml of dichloromethane, 9.144g (0.048mol, 1.2eq) of p-toluenesulfonyl chloride, 6.06g (0.06mol, 1.5eq) of triethylamine, a small amount of 4-dimethylaminopyridine into The reaction takes about 3 hours; then, add appropriate amount of water to terminate the reaction, extract with ethyl acetate, dry, and spin to dry, to obtain a white solid (1S, 2S)-3-(dimethylamino)-2-methyl-1-( 3-tert-Butyldimethylsiloxyphenyl)propyl-4-toluenesulfonate, the two-step yield is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com