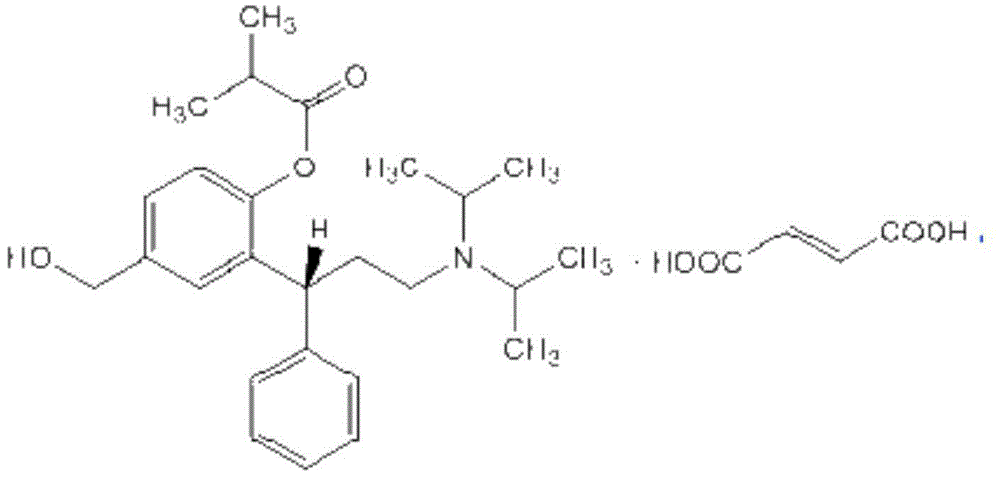
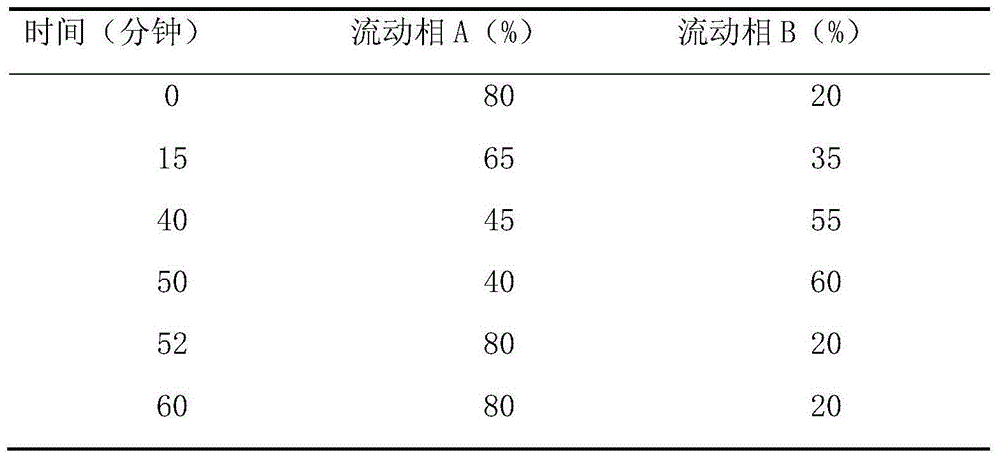
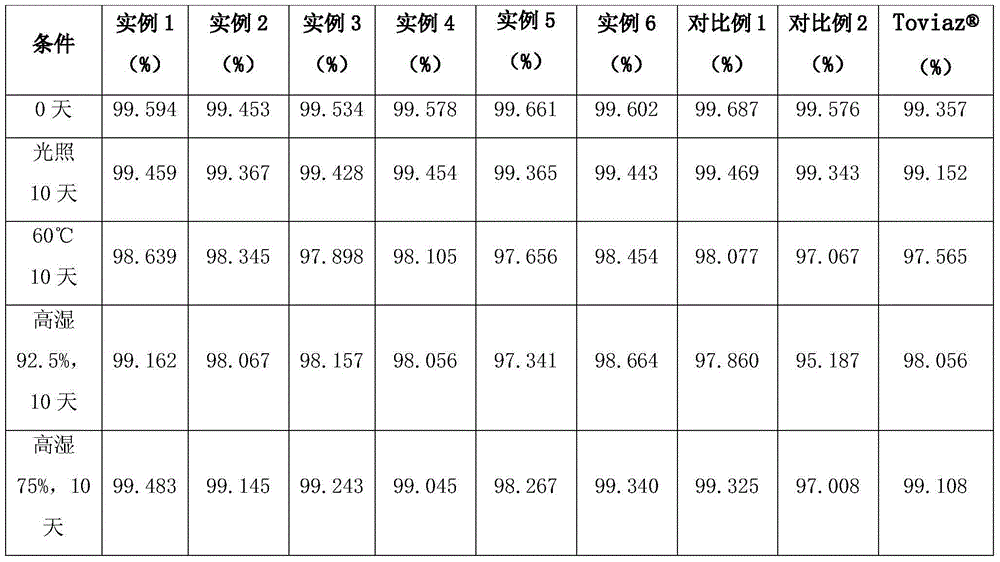
Pharmaceutical composition containing fesoterodine and preparation method thereof
A fesoterodine and composition technology, applied in the field of fesoterodine-containing pharmaceutical composition and its preparation, can solve the problems of fesoterodine's easy decomposition, gastrointestinal side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] With dextrin as stabilizer, prepare fesoterodine fumarate sustained-release tablet, its prescription is shown in Table 1 (1000 tablet quantity):
[0042] Table 1
[0043] Fesoterodine fumarate
4g
120g
65g
25g
Hypromellose K15M
50g
Hypromellose K100M
50g
15g
[0044] Its preparation method is as follows:
[0045]Weigh the raw and auxiliary materials according to Table 1, mix fesoterodine fumarate and dextrin, pass through a 100-mesh sieve and mix evenly, then mix with lactose, microcrystalline cellulose, hypromellose and pass through a 100-mesh sieve to mix uniform. Mix the powder and add 95% ethanol as a wetting agent to make a soft material, pass through a 20-mesh sieve to make wet granules, and dry the wet granules at 45°C until the water content is less than 4.0%, to obtain dry granules. The dry granules are passed thro...
Embodiment 2
[0048] Do stabilizer with maltodextrin, prepare fesoterodine fumarate sustained release tablet, its prescription is shown in Table 2 (quantity of 1000):
[0049] Table 2
[0050] Fesoterodine fumarate
4g
100g
65g
25g
Hypromellose K15M
50g
Hypromellose K100M
50g
9g
[0051] Its preparation method is as follows:
[0052] Refer to Table 2 to weigh the raw and auxiliary materials, mix fesoterodine fumarate and maltodextrin, pass through a 120-mesh sieve and mix evenly, then mix with lactose, microcrystalline cellulose, hypromellose and mix with a 40-mesh sieve Uniform; mixed with powder, 80% ethanol as wetting agent, made into soft material, passed through 14 mesh sieve to make wet granules, dried at 45°C until the moisture content was less than 4.0%, to obtain dry granules. The dry granules are passed through a 14-mesh sieve for gran...
Embodiment 3
[0055] Make stabilizer with mannitol, prepare fesoterodine fumarate sustained-release tablet, its prescription is shown in Table 3 (1000 tablet quantities):
[0056] table 3
[0057] Fesoterodine fumarate
4g
80g
lactose
65g
25g
Hypromellose K15M
50g
Hypromellose K100M
50g
12g
3g
[0058] Its preparation method is as follows:
[0059] Refer to Table 3 to weigh the raw and auxiliary materials, mix fesoterodine fumarate and mannitol, pass through a 40-mesh sieve and mix evenly, then mix with lactose, microcrystalline cellulose, hypromellose and mix evenly with a 100-mesh sieve ; Mix the powder and add 100% ethanol as a wetting agent to make soft materials, pass through a 20-mesh sieve to make wet granules, and dry the wet granules at 45°C until the water content is less than 4.0%. Obtain dry granules; pass the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com