Preparation method of 4-(4-phenylbutoxy) benzoic acid
A technology of phenylbutoxy and benzoic acid, applied in the field of preparation of 4-benzoic acid, can solve problems such as being unsuitable for large-scale industrial production, expensive phenylbutanol, complicated process, etc., and achieves short synthetic route and novel route. , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
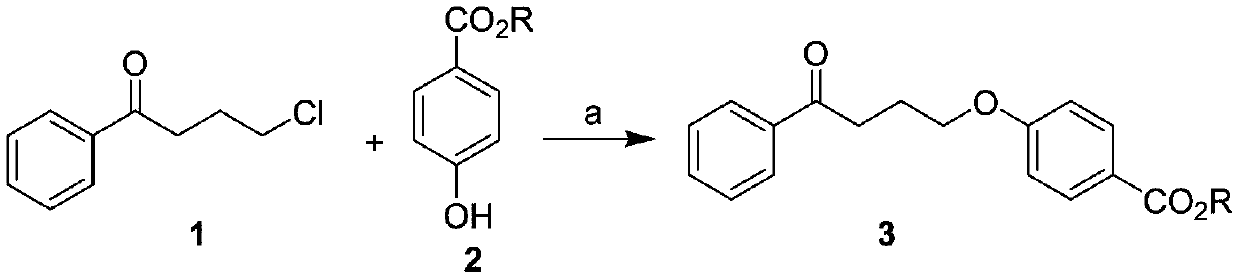
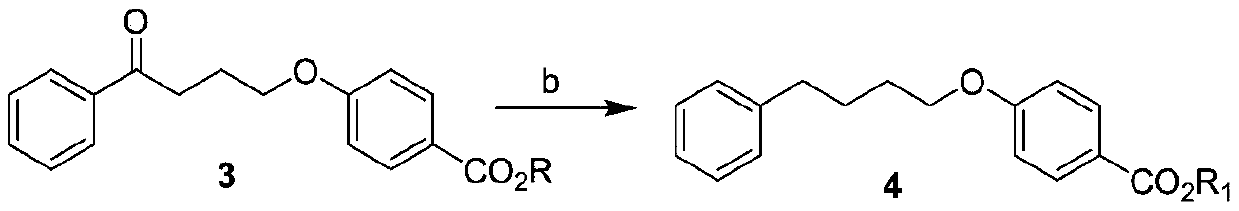
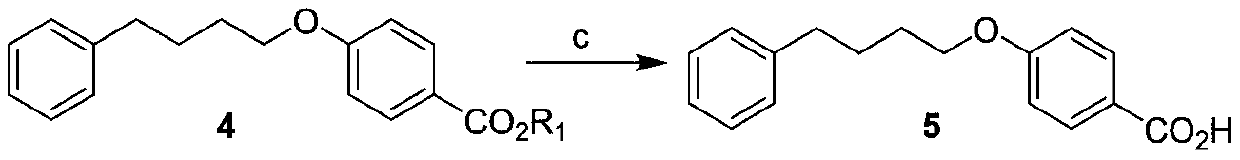
[0038] This example provides a preparation method of 4-(4-phenylbutoxy)benzoic acid, which is specifically carried out according to the following steps, and the compound γ-chlorobutyrophenone is prepared according to the literature method.
[0039] 1. Synthesis of compound 3a: methyl 4-(4-oxo-4-phenylbutoxy)benzoate
[0040] Weigh 9.1 grams of γ-chlorobutanone and 8.4 grams of methyl p-hydroxybenzoate, dissolve them in 100 milliliters of DMF, add 27.6 grams of potassium carbonate, stir, heat up, stir at 100°C for 5 hours, cool down, filter, and decompress the filtrate Concentrate, dissolve in dichloromethane, wash with 1N sodium hydroxide, separate the organic phase, dry over anhydrous sodium sulfate, filter and concentrate to obtain a white solid that is directly used in the next reaction.
[0041] Reaction formula:
[0042]
[0043] 2. Synthesis of compound 3b: benzyl 4-(4-oxo-4-phenylbutoxy)benzoate
[0044] According to the method in the first step, benzyl p-hydroxybe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com