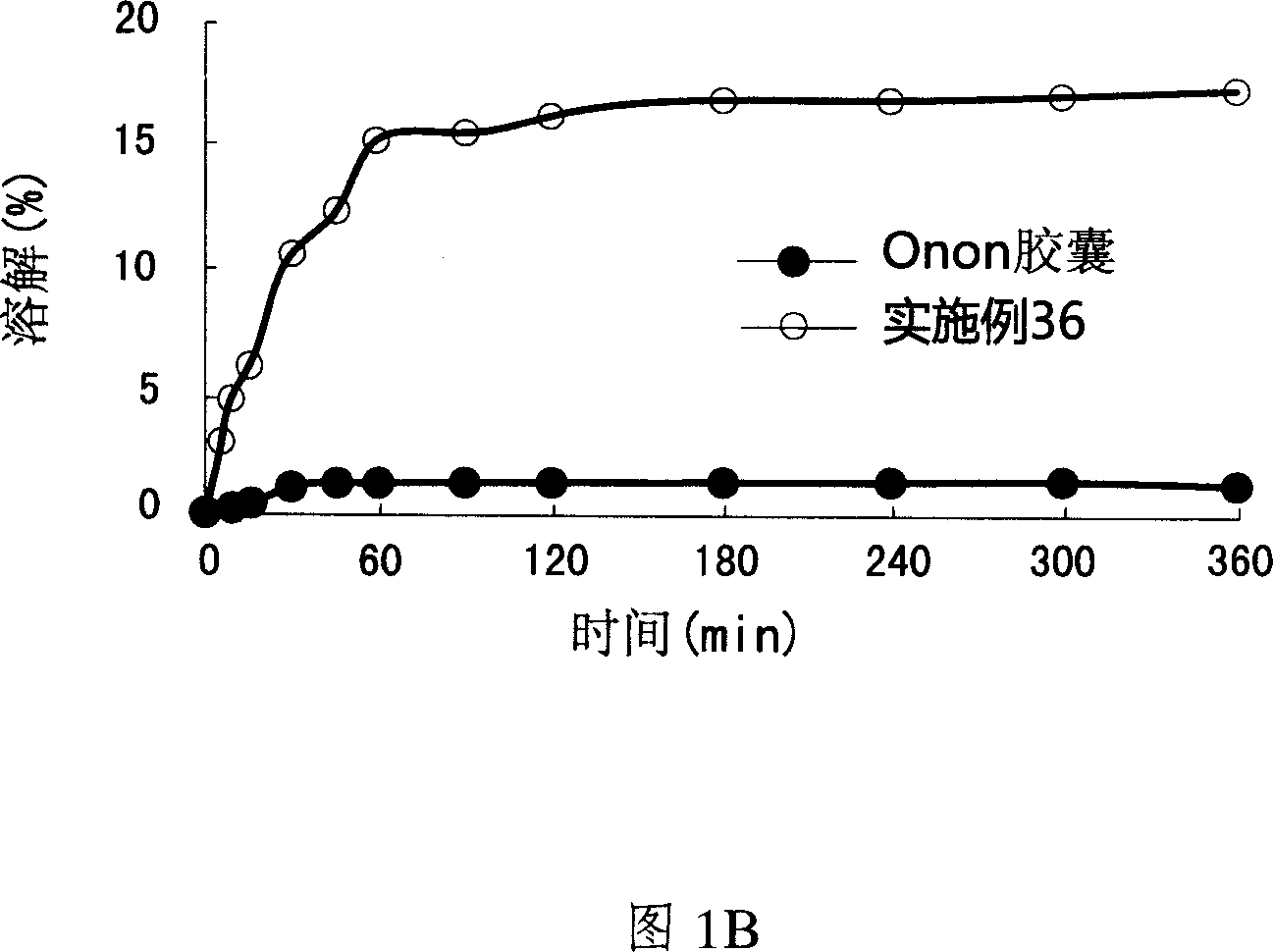
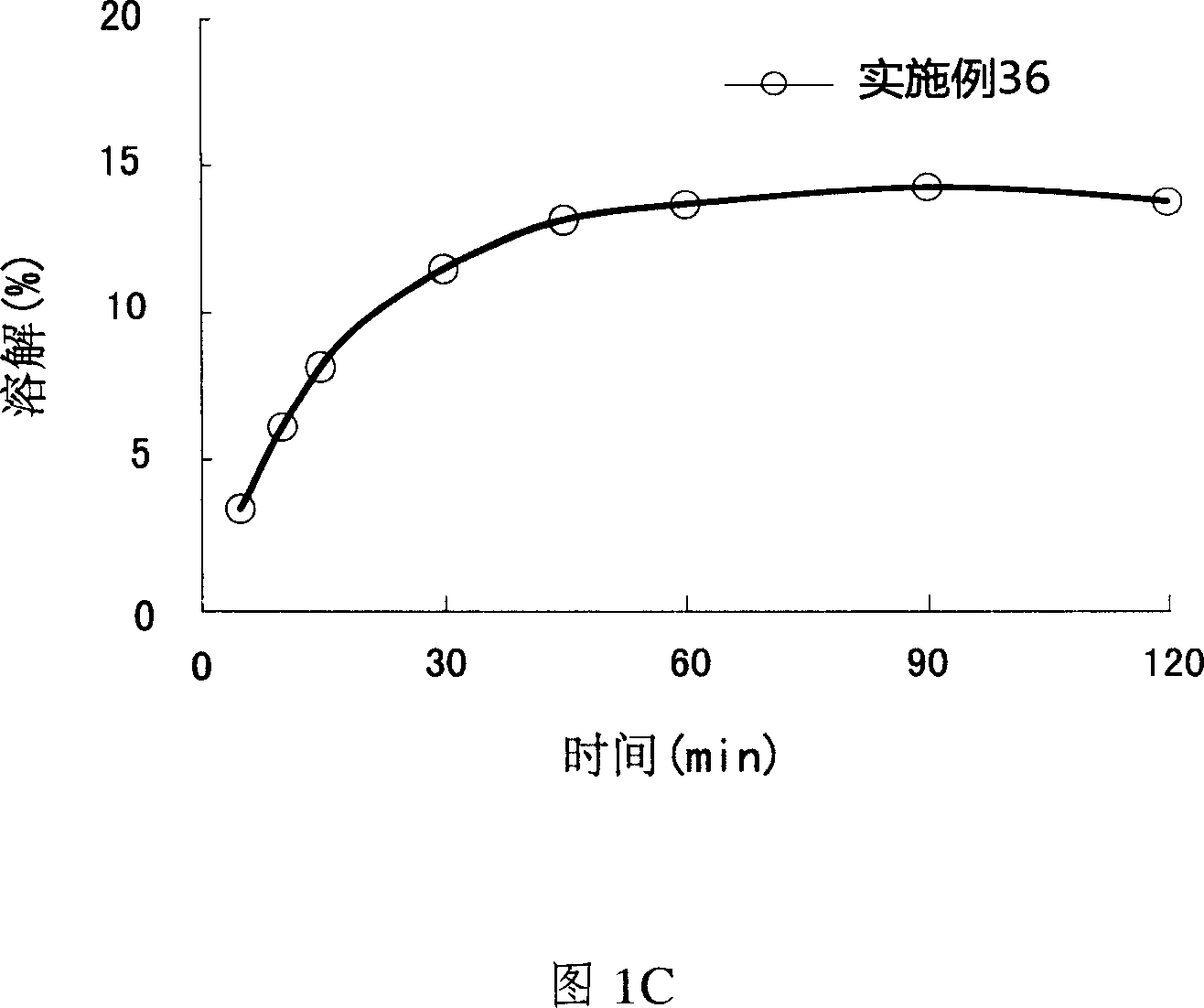
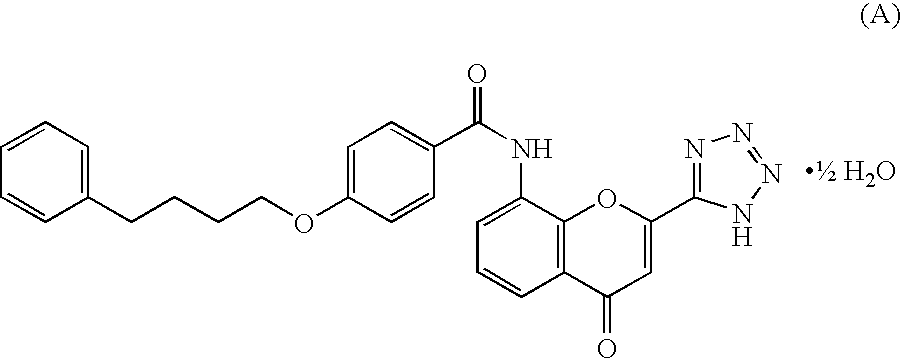
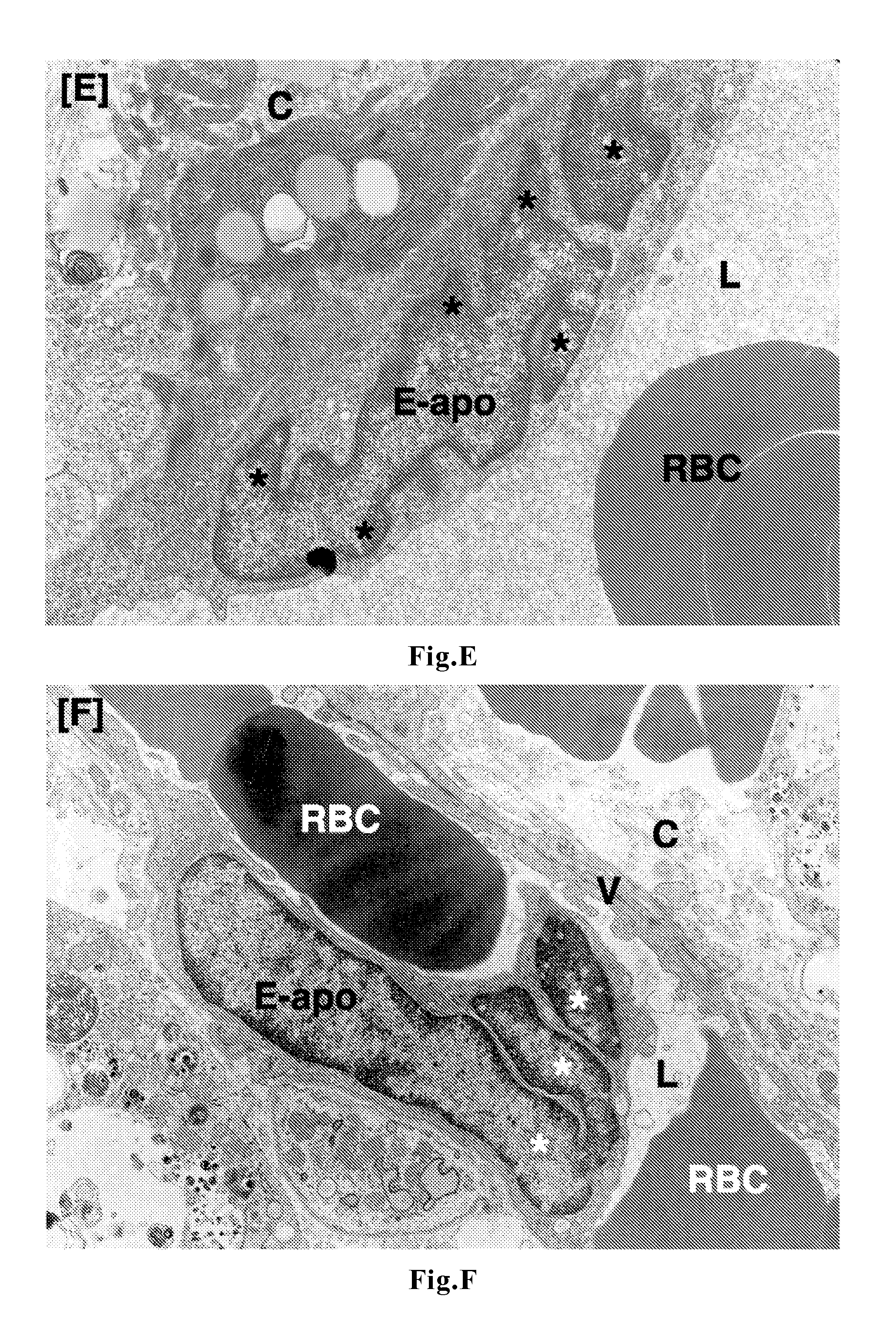
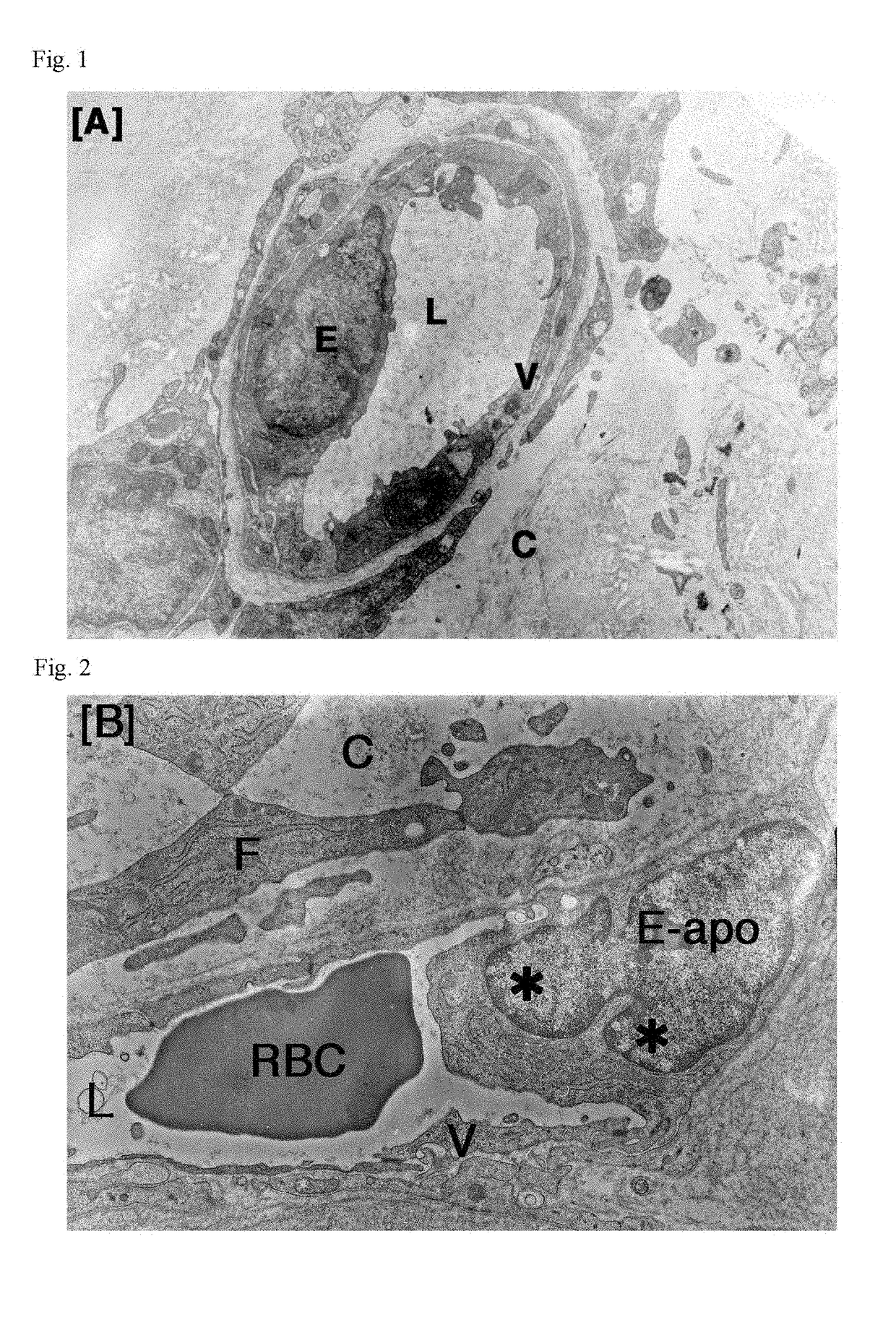
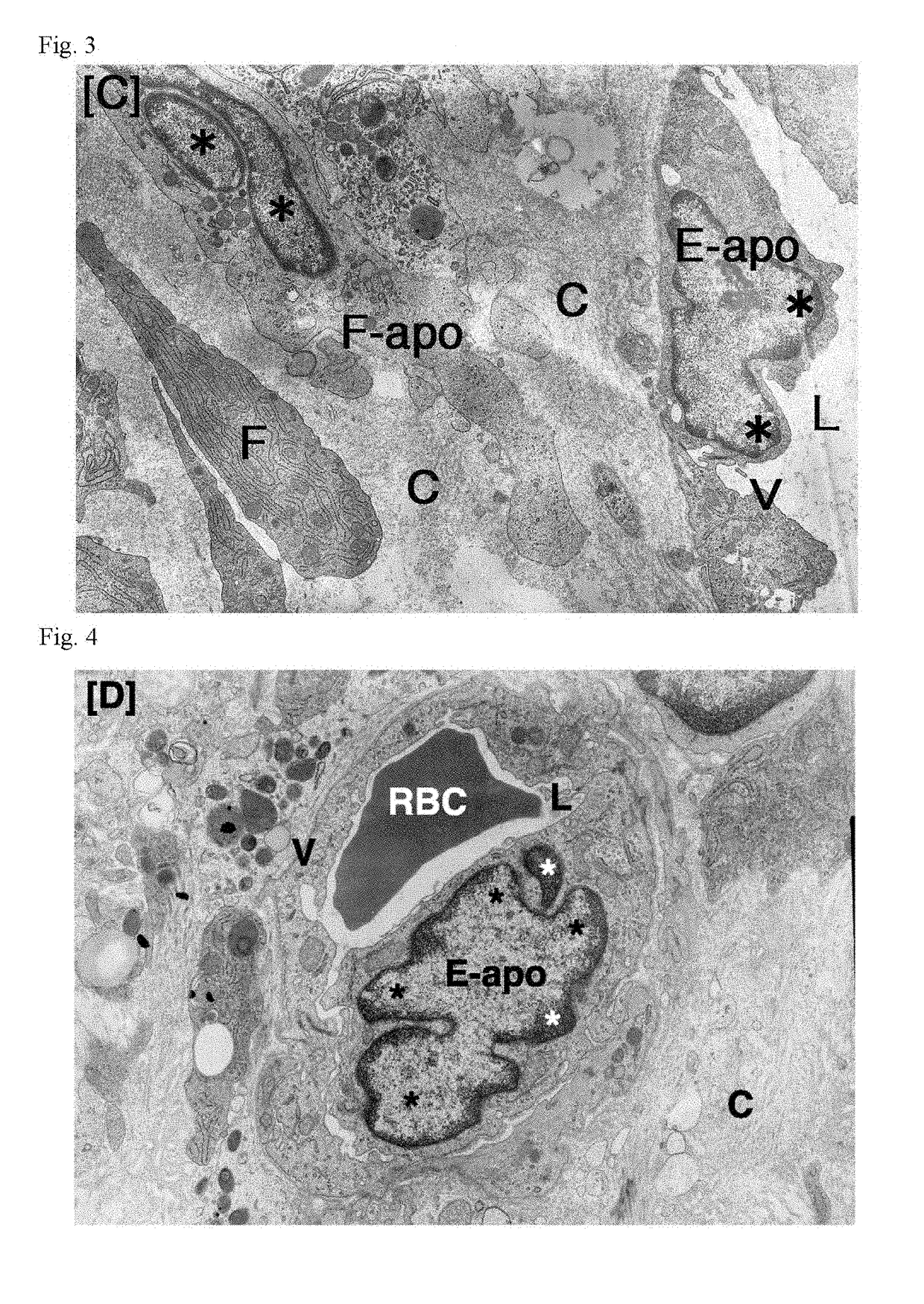
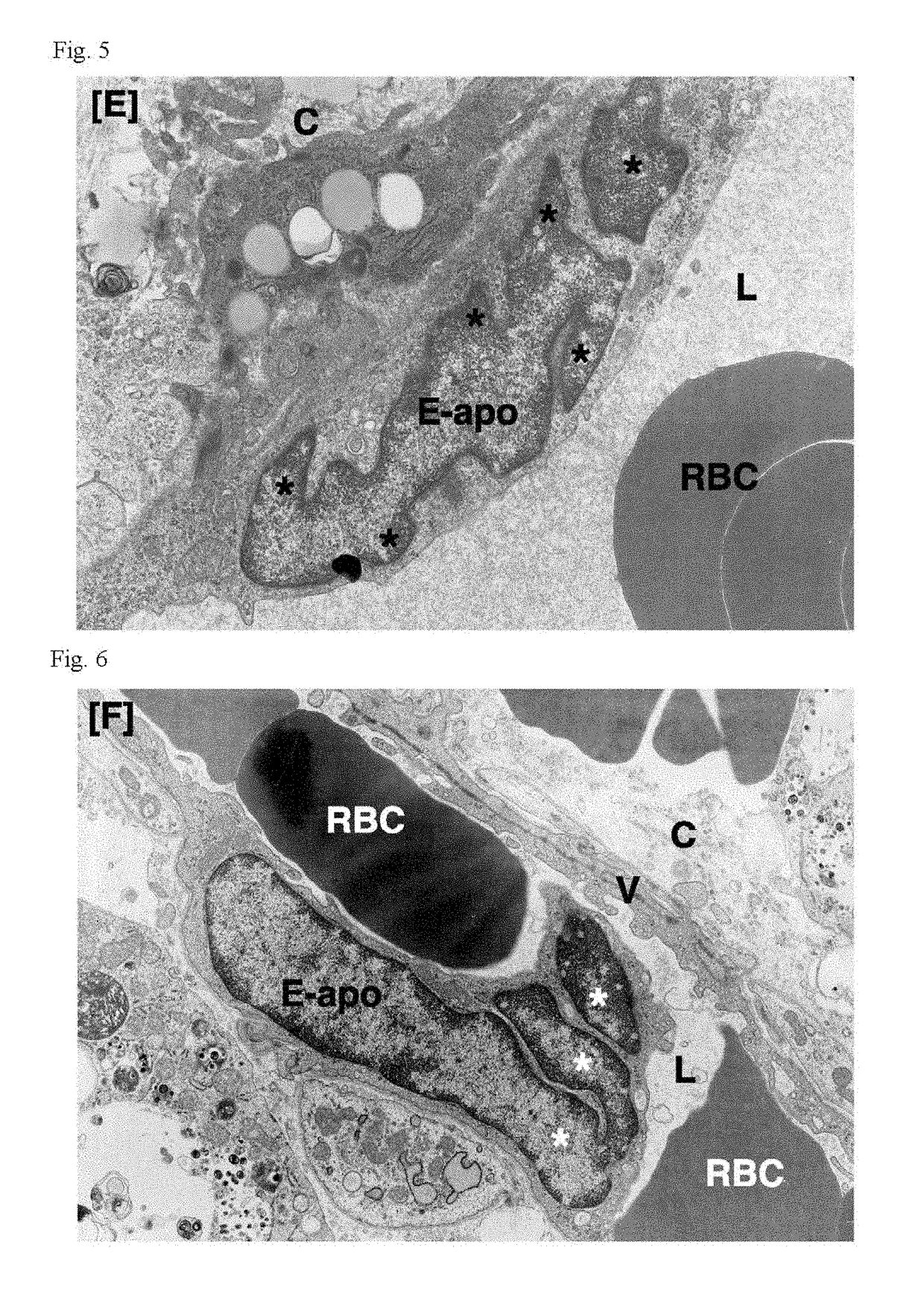
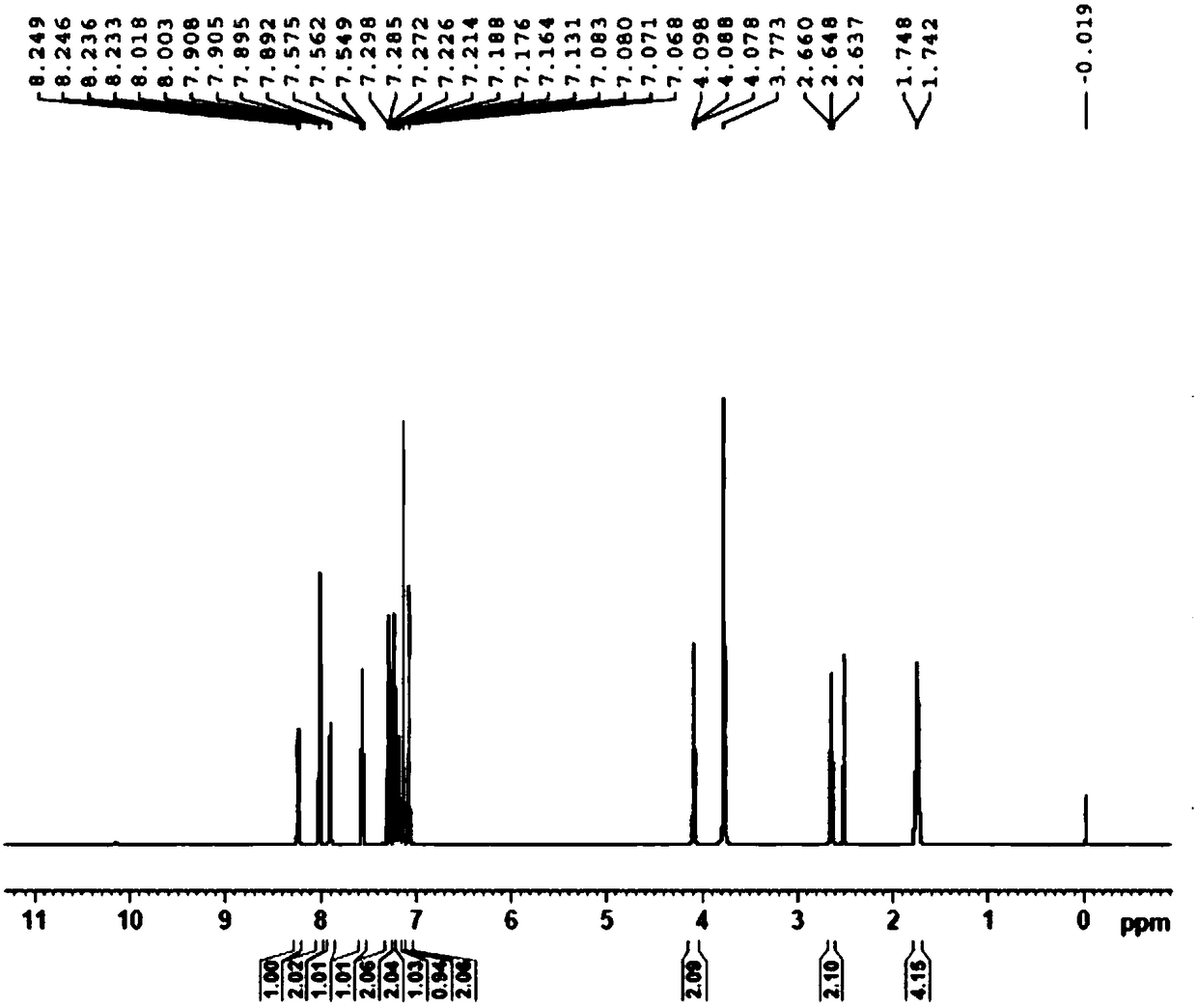
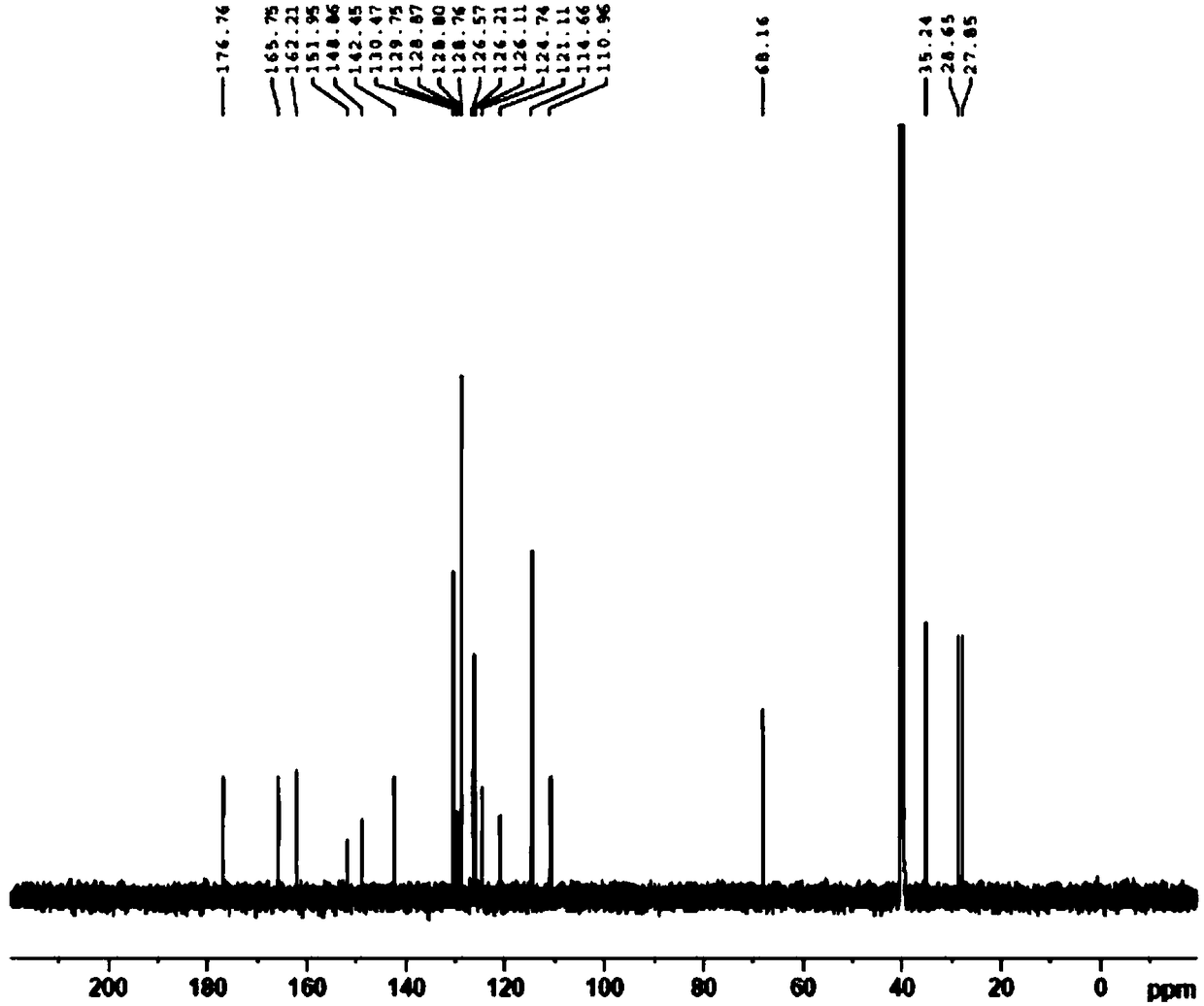
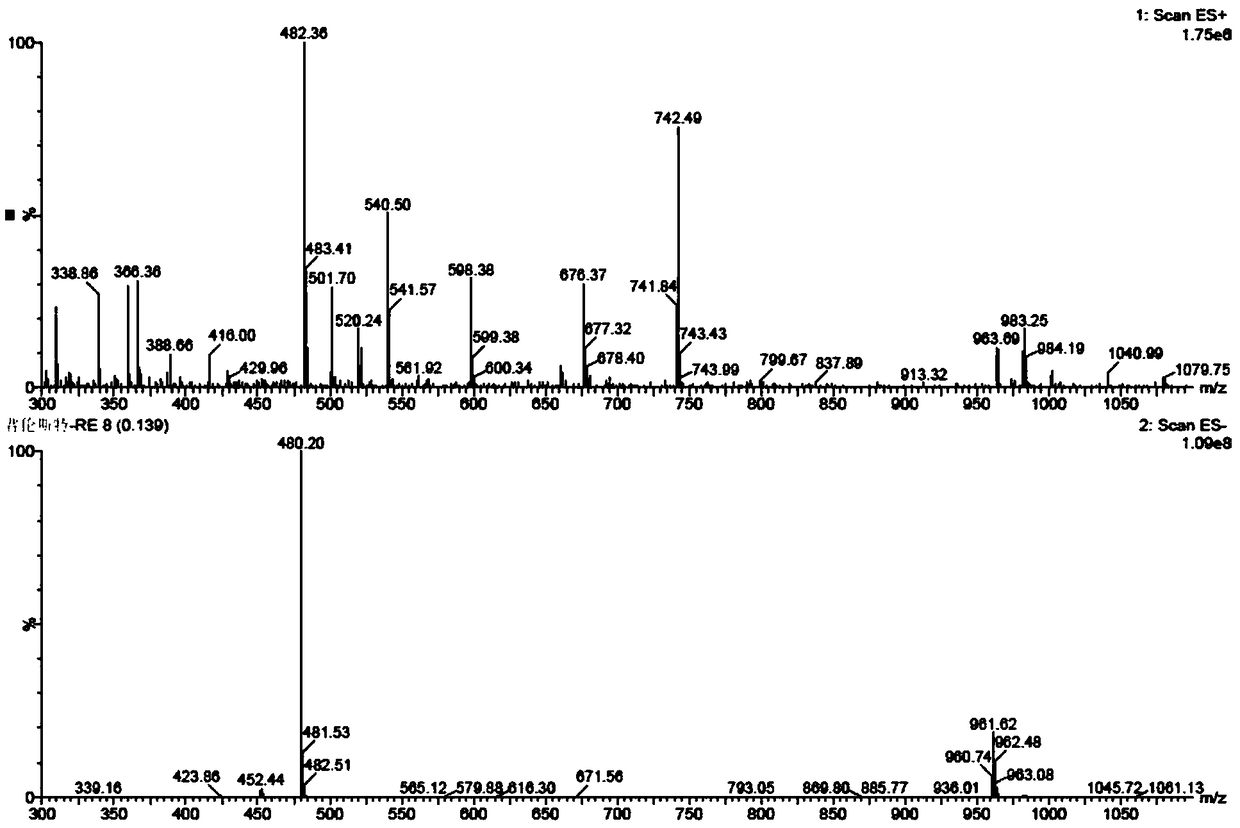
Patents
Literature
31 results about "Pranlukast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
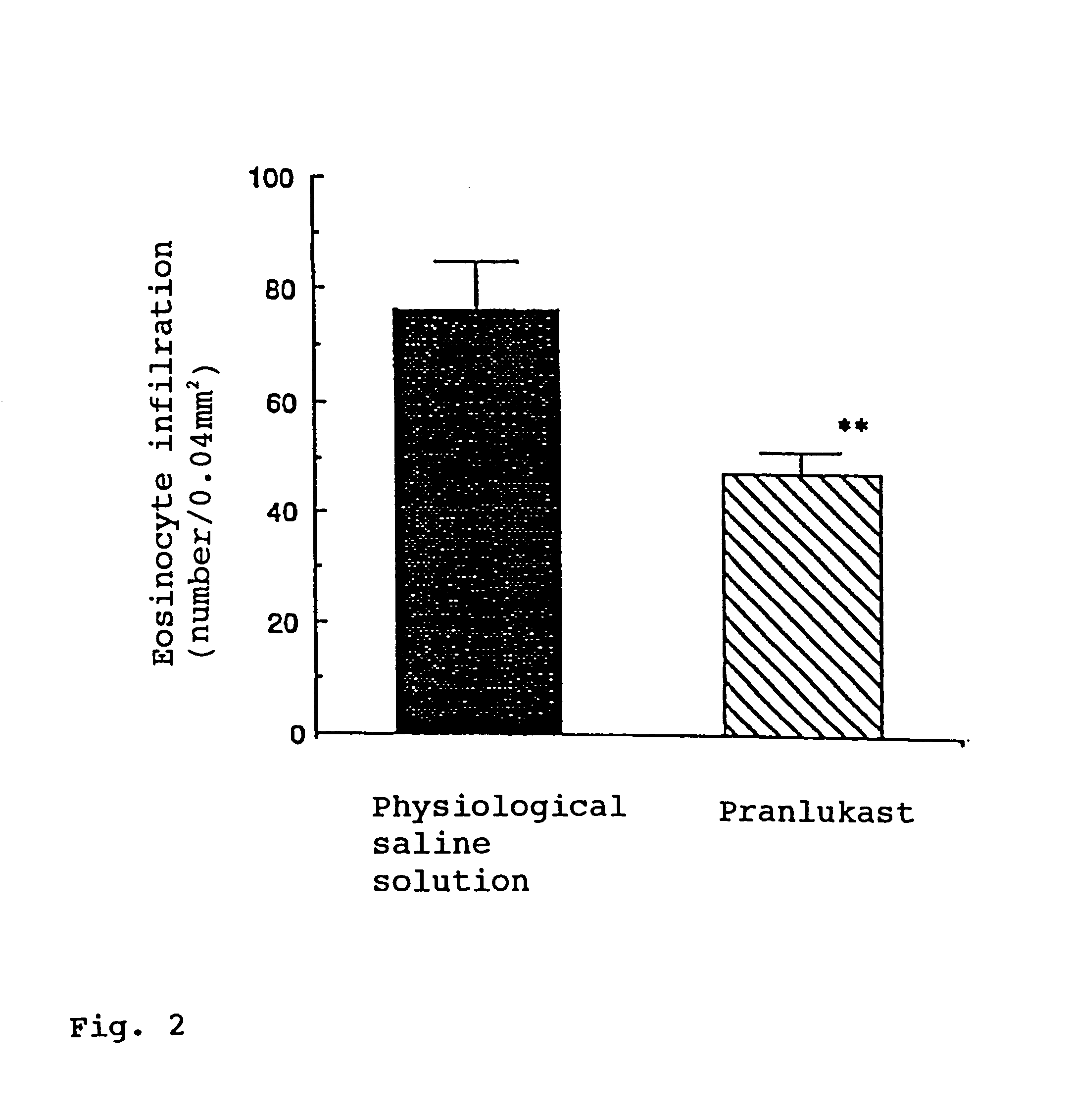
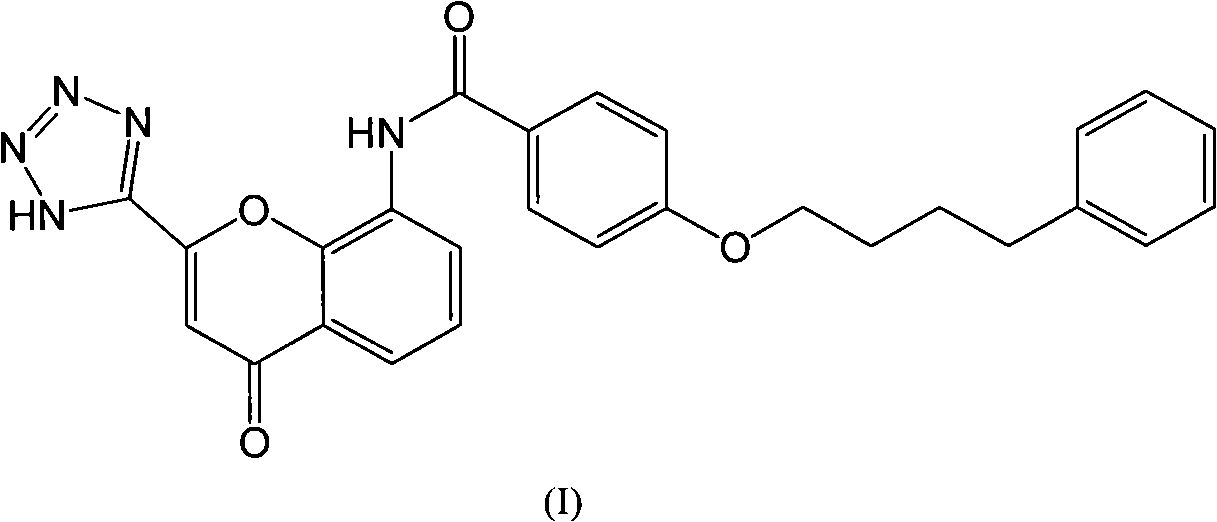
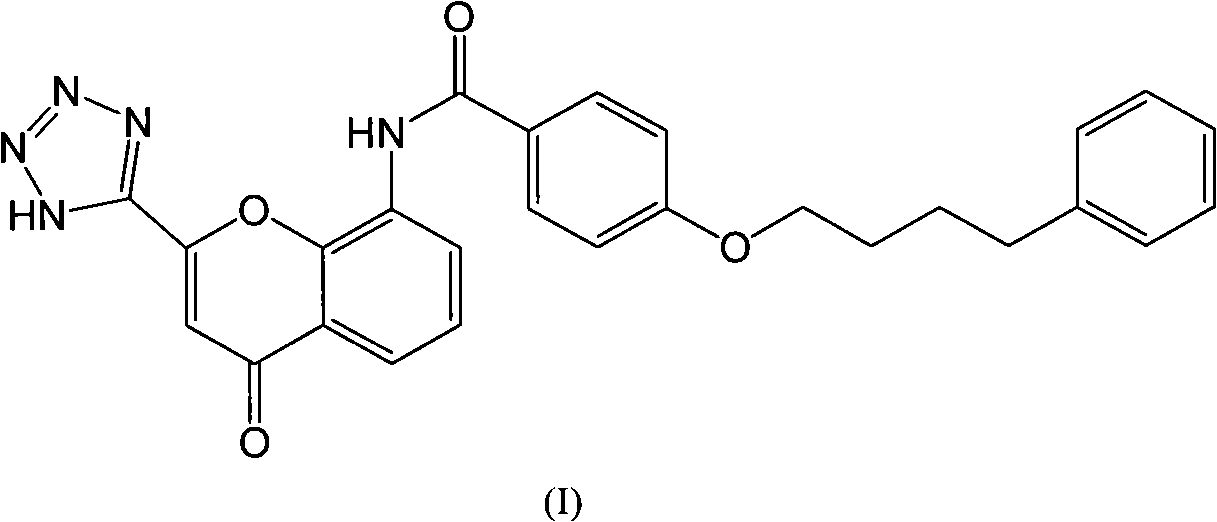
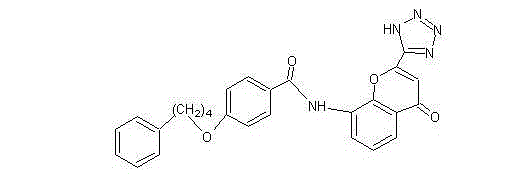
Pranlukast (brand name Onon, オノン) is a cysteinyl leukotriene receptor-1 antagonist. This drug works similarly to Merck & Co.'s montelukast (Singulair). It is widely used in Japan. Medications of this class, which go under a variety of names according to whether one looks at the American, British or European system of nomenclature, have as their primary function the antagonism of bronchospasm caused, principally in asthmatics, by an allergic reaction to accidentally or inadvertently encountered allergens.
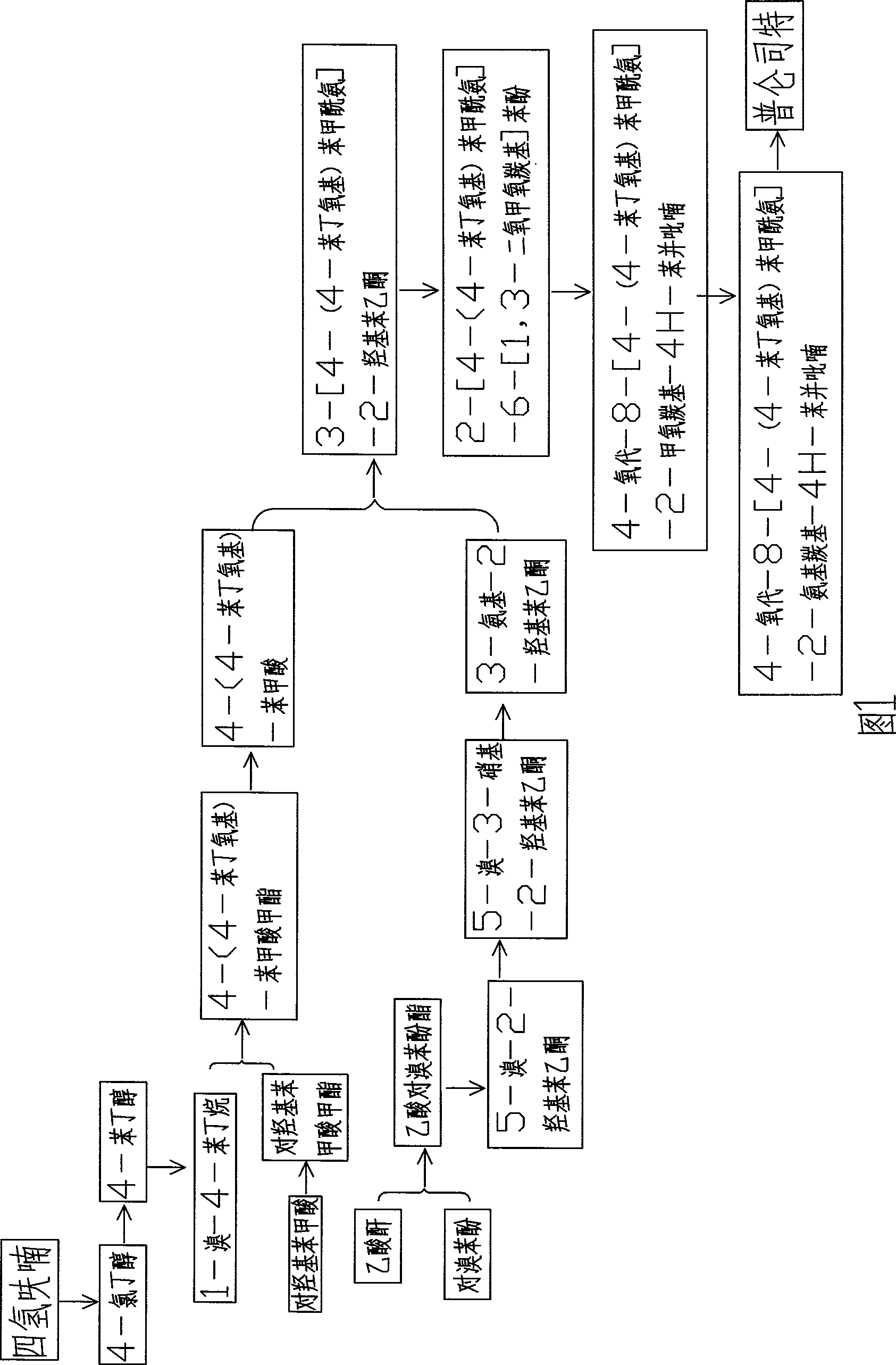
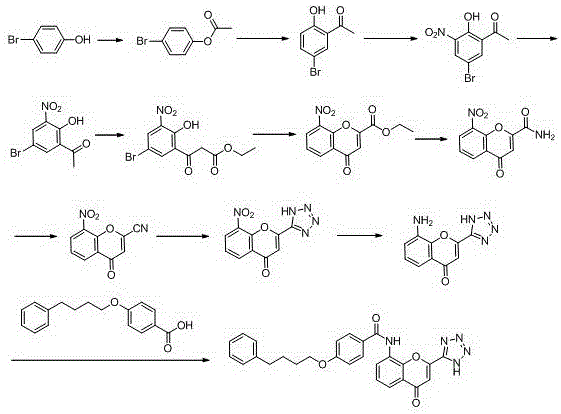
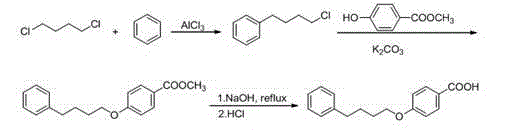
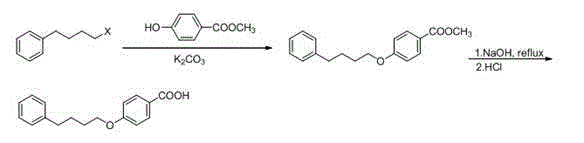
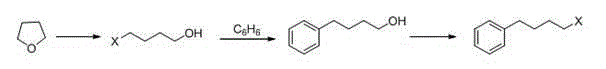
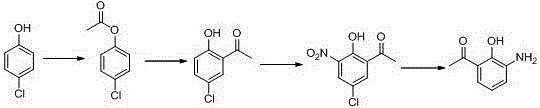
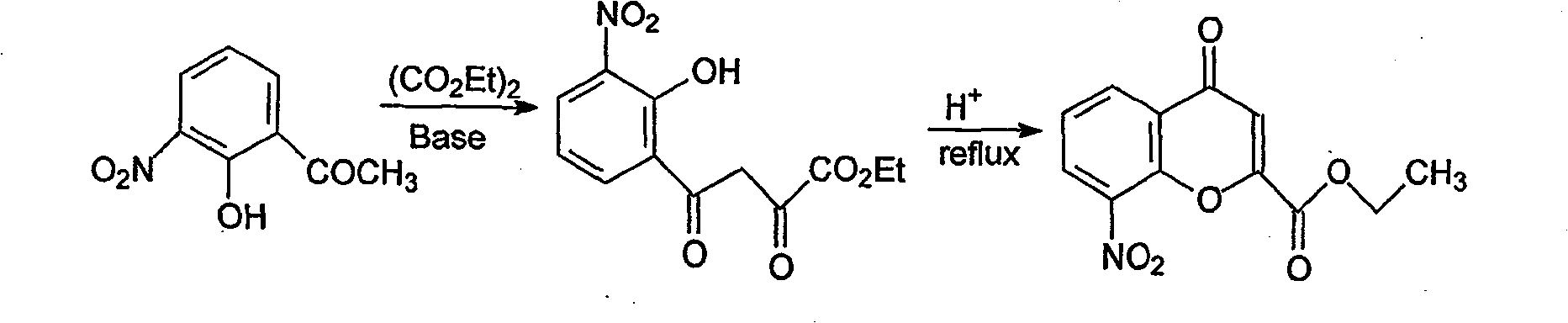
Method for synthesizing drug pranlukast from tetrahydrofuran path
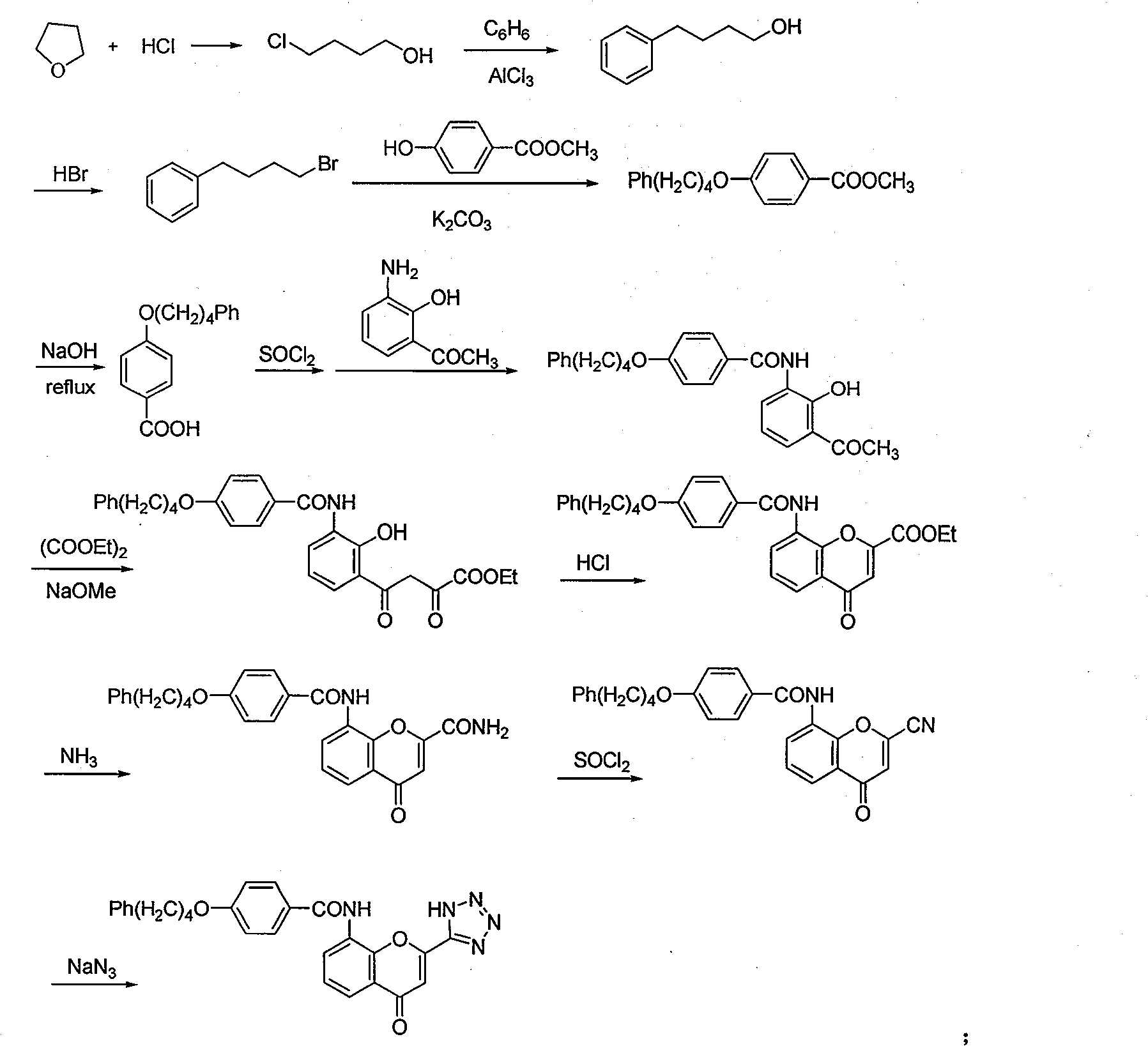
ActiveCN101450943AOvercoming the difficulty of synthesis and the shortcomings of not being easy to industrializeImprove responseOrganic chemistryAlkyl transferTetrahydrofuran
The invention discloses a new method for synthesizing pranlukast, in particular to a method for synthesizing medicinal pranlukast from tetrahydrofuran. The method takes the tetrahydrofuran as raw materials to prepare the pranlukast through ring opening, Friedel-Crafts alkylation, bromination, condensation and ring closing reactions. The method has the characteristics of readily available raw materials, simple reaction for preparation of an intermediate, high yield, safe reactions and suitability for industrial production, and can be used to prepare the medicinal pranlukast for treating asthma and allergic rhinitis.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Aqueous liquid pharmaceutical composition containing as main component benzopyran derivative
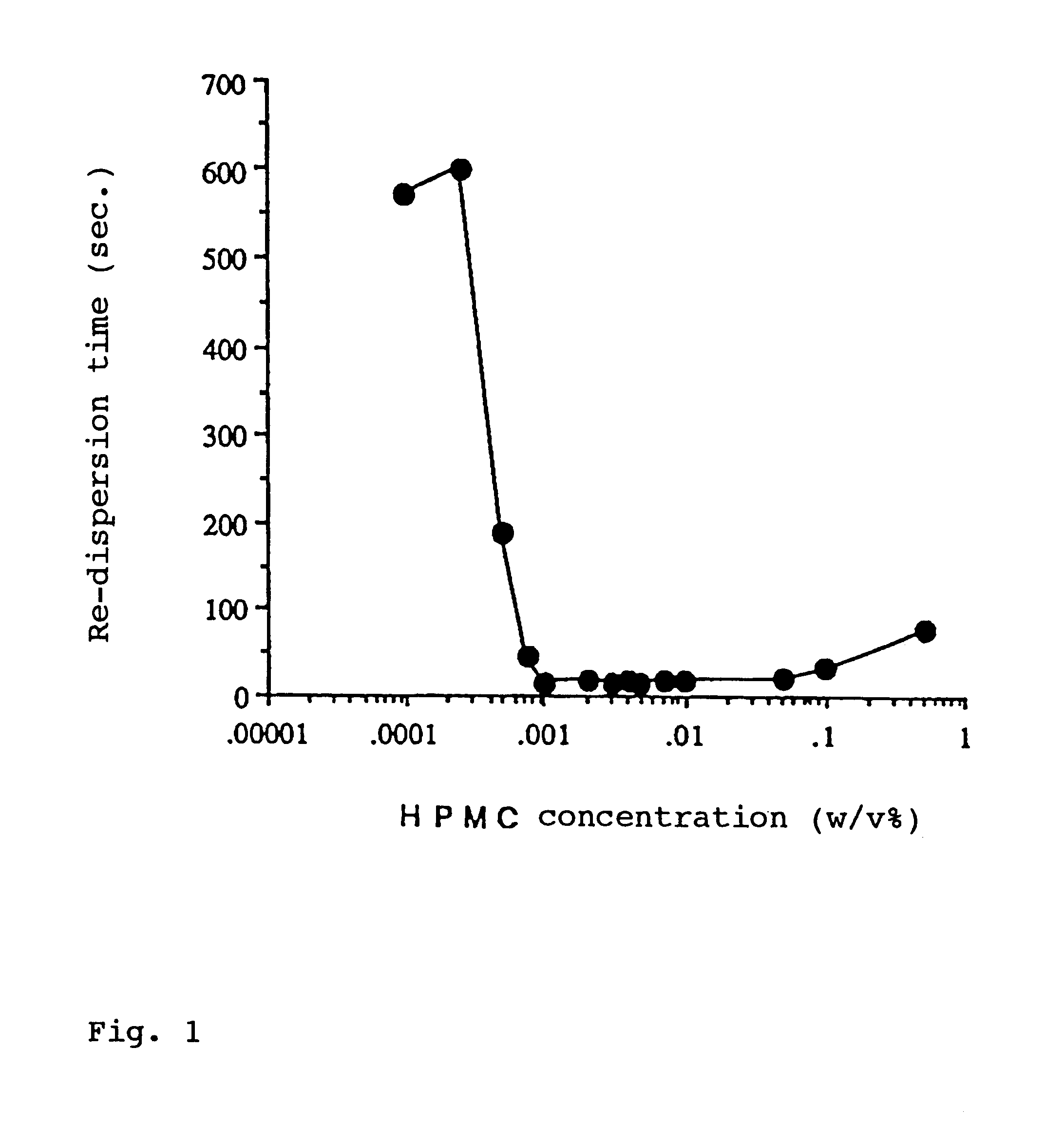
InactiveUS6274609B1Enhanced inhibitory effectPromote solubilization and suspensionBiocideSenses disorderBenzopyranHigh concentration
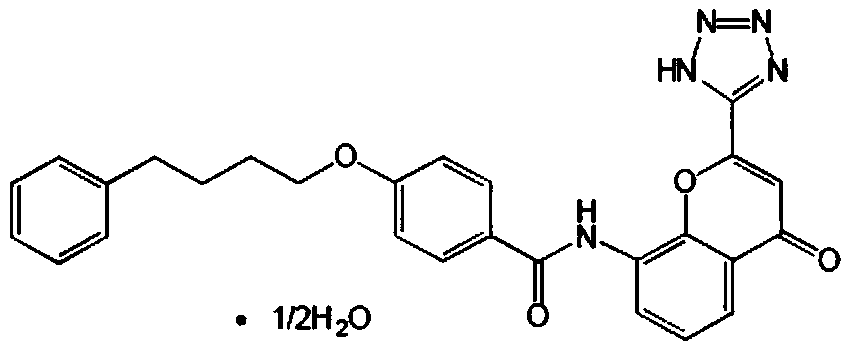
In order to promote solubilization or suspension of 4-oxo-8-[4-(4-phenylbutoxy)benzoylamino]-2-(tetrazol-5-yl)-4H-1-benzopyran or its hydrate (pranlukast) in water, at least one component selected from surfactants, water-soluble cellulose derivatives and water-soluble vinyl polymers is formulated together with pranlukast. Thus, it is possible to provide an aqueous liquid pharmaceutical composition containing higher concentration of pranlukast and having good properties.
Owner:ONO PHARMA CO LTD +1
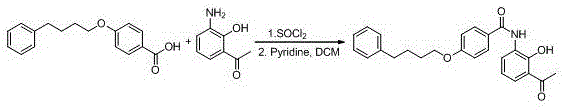
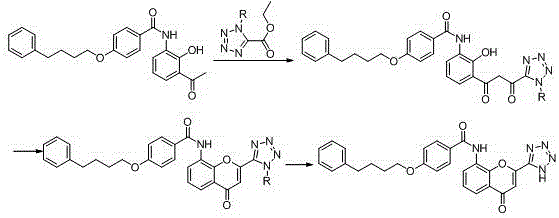
New preparation method of Pranlukast
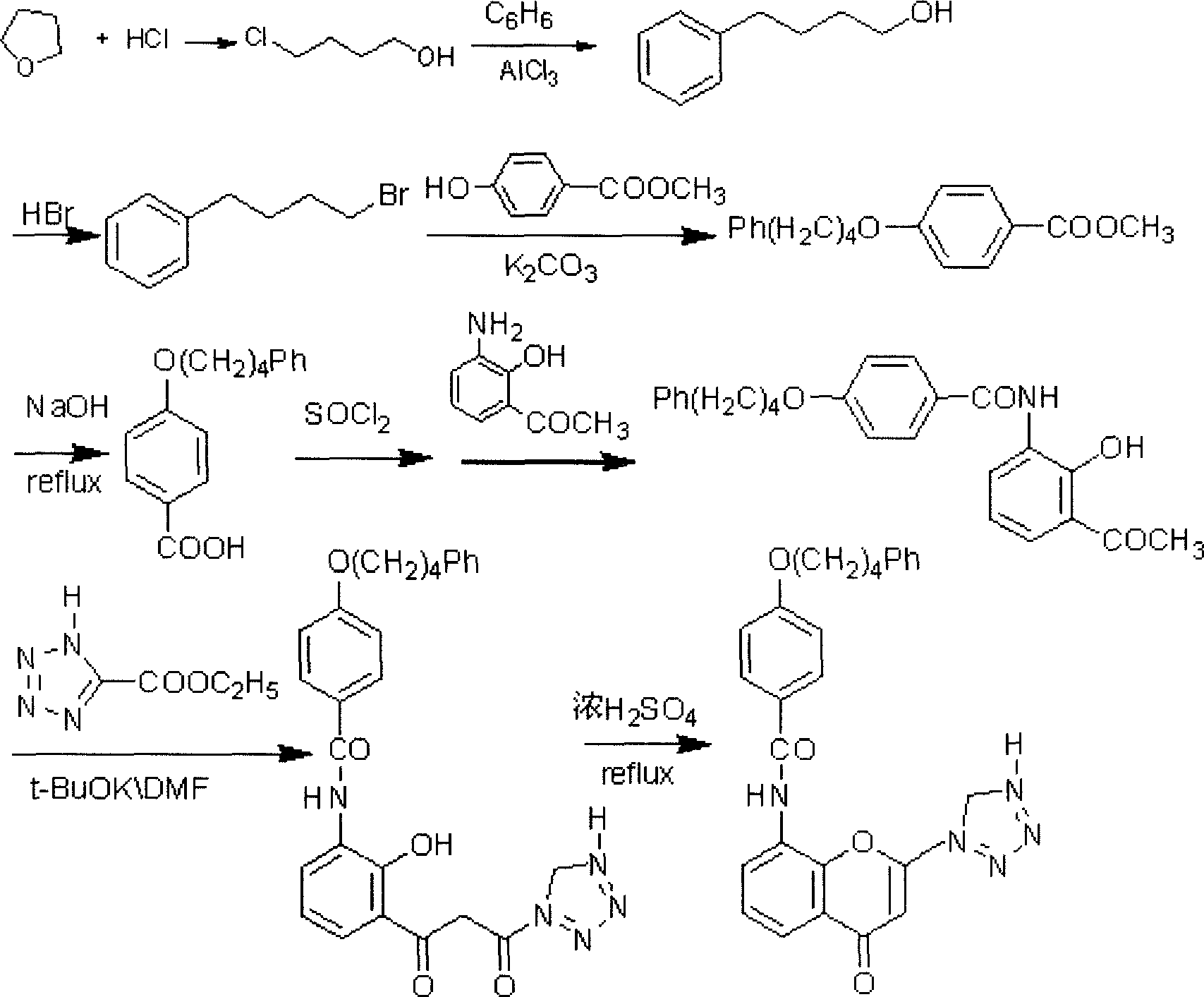
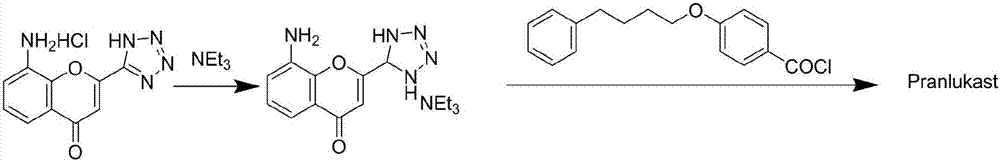
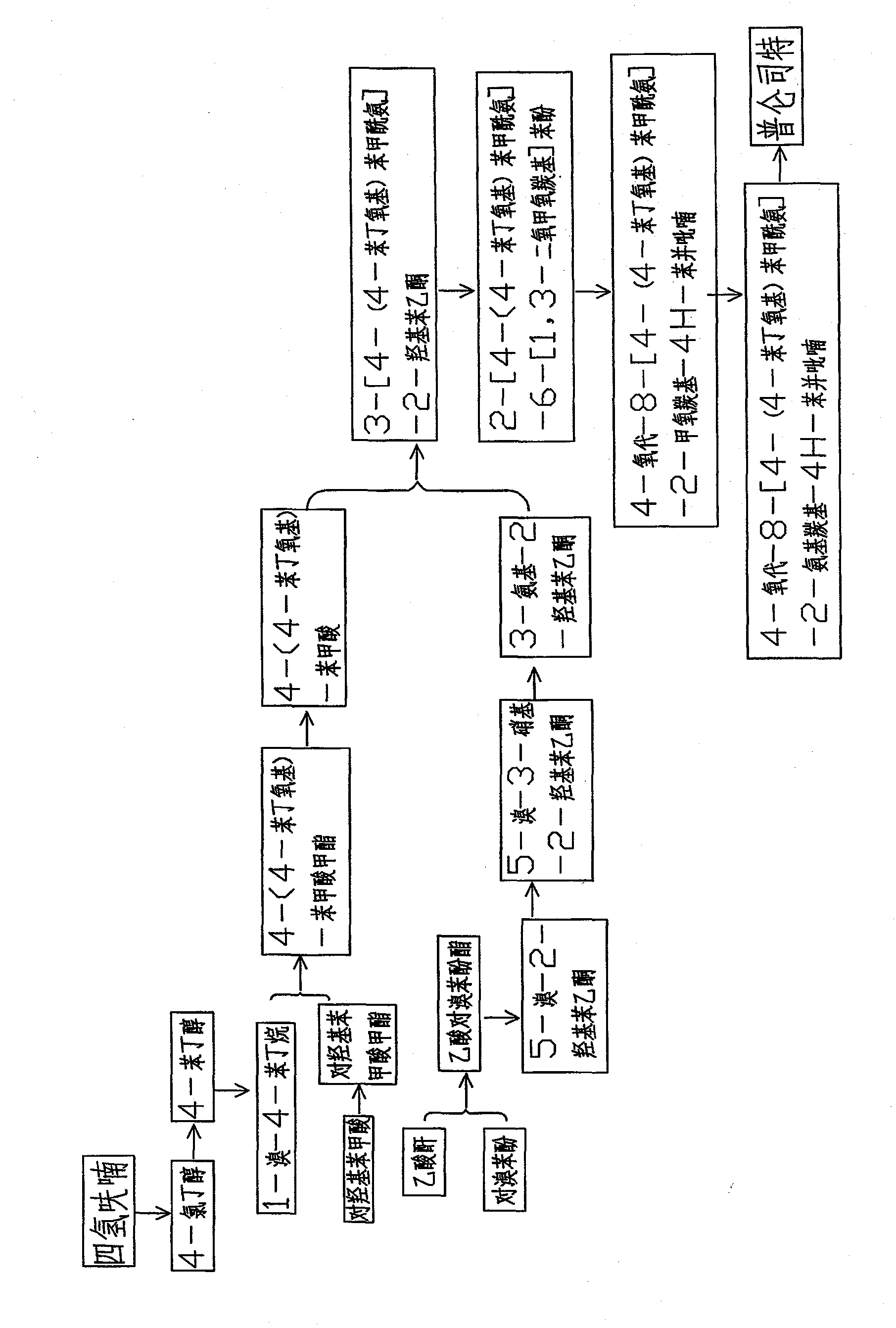
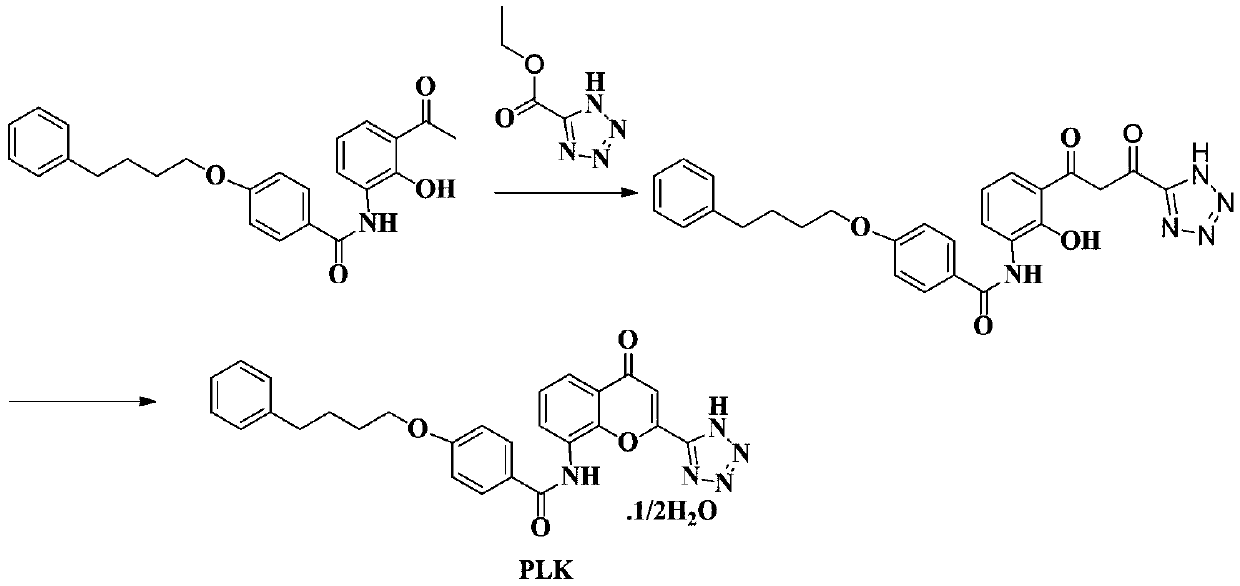
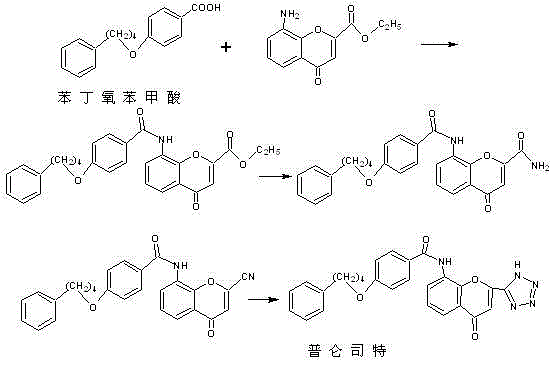
The invention provides a new preparation method of drug Pranlukast for treating asthma. The new preparation method includes the specific steps that with 2-aminophenol-4-sulfonic acid as a starting material, a key intermediate 3-amino-2-hydroxyacetophenone is prepared by means of acylation, Fries rearrangement and deprotection, then reacts with 4-(phenylbutoxy)benzoic acid, and then is subjected to condensation with ethyl 1H-tetrazole-5-acetate, and finally preparation is achieved through ring closing under the acidic condition. Compared with the prior art, the raw material used for the new preparation method is low in price and easy to obtain, industrialization of a process can be achieved easily, and the obtained final product is high in purity; and no dangerous process exists, equipment is simple, and the route is novel.
Owner:上海微巨实业有限公司
Novel process for synthesizing Pranlukast intermediate p-phenylbutoxybenzoic acid
InactiveCN104387268ARaw materials are cheap and easy to getShort process routeOxygen-containing compound preparationOrganic compound preparationAlkyl transferEnvironmental resistance
The invention discloses a novel process for synthesizing Pranlukast intermediate p-phenylbutoxybenzoic acid, belonging to the field of medicinal chemistry. The process is characterized by comprising the following steps: by using low-price 1,4-dichlorobutane as an initial raw material, carrying out reactions such as Friedel-Crafts alkylation, substitution and hydrolysis, thereby obtaining the p-phenylbutoxybenzoic acid. The method has the characteristics of cheap and easily available raw materials, short process route, low cost, reaction safety, environment friendliness and the like and is suitable for industrial production.
Owner:UNIV OF JINAN
Composition for treating respiratory and skin diseases, comprising at least one leukotriene antagonist and at least one antihistamine
A pharmaceutical composition useful in the treatment of sneezing, itching runny nose, nasal congestion, redness of the eye, tearing, itching of the ears or palate, shortness of breath, inflammation of the bronchial mucosa, reduced Forced Expiratory Volume In One Second (FEV1), coughs, rash, itchy skin, headaches, and aches and pains associated with seasonal allergic rhinitis, perennial allergic rhinitis, common colds, otitis, sinusitus, allergy, asthma, allergic asthma and / or inflammation, in a mammalian organism in need of such treatment. The composition comprises: i) an effective amount of at least one leukotriene antagonist selected from a) montelukast, b) 1-(((R)- (3-(2-(6,7- difluoro-2- quinolinyl)ethenyl) phenyl)-3-(2- (2-hydroxy-2- propyl)phenyl) thio)methylcyclopropaneacetic acid; c) 1-(((1(R)-3 (3-(2-(2,3- dichlorothieno[3, 2-b]pyridin-5-yl) -(E)-ethenyl)phenyl) -3-(2-(1-hydroxy-1- methylethyl) phenyl)propyl) thio)methyl) cyclopropaneacetic acid; d) pranlukast; or f) [2-[[2-(4-tert -butyl-2-thiazolyl) -5-benzofuranyl] oxymethyl]phenyl] acetic acid; or a pharmaceutically acceptable salt thereof; in admixture with ii) an effective amount of at least one antihistamine which is descarboethoxyloratidine, cetirizine, fexofenadine, ebastine, astemizole, norastemizole, epinastine, efletirizine or a pharmaceutically acceptable salt thereof.
Owner:SCHERING AG
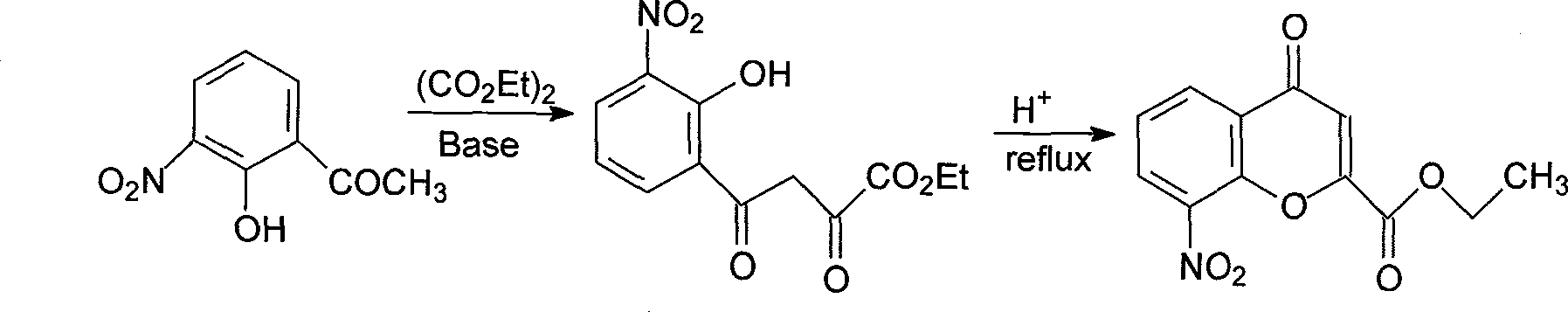
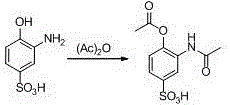
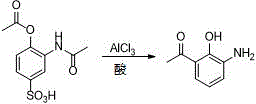
New preparation method of 3-amino-2-hydroxyphenylacetone
ActiveCN106831457ACheap and easy to getHigh purityOrganic compound preparationSulfonic acid preparationState of artFries rearrangement
The invention discloses a new preparation method of a key intermediate, namely 3-amino-2-hydroxyphenylacetone, for preparation of Pranlukast. The new preparation method comprises the following main steps: taking 2-aminophenol-4-sulfonic acid as a starting raw material, and carrying out acylation, Fries rearrangement, hydrolysis and deprotection, so that 3-amino-2-hydroxyphenylacetone is obtained. Compared with the prior art, the new preparation method disclosed by the invention has the advantages that the used raw materials are cheap and easily available, technology can easily realize industrialization, and the obtained final product is high in purity; no danger technology is adopted, and equipment is simple; and route is novel, and synthesis route is short.
Owner:上海微巨实业有限公司
Synthesis technology of intermediate of anti-asthma drug namely pranlukast
InactiveCN104402711ALow costMild reaction conditionsPreparation from carboxylic acid saltsOrganic compound preparationBenzoic acidCombinatorial chemistry
The invention relates to a synthesis technology of an intermediate [4-(4-phenylbutoxy)benzoic acid] of an anti-asthma drug namely pranlukast. The synthesis technology comprises the following steps: (1) carrying out etherification reactions; (2) performing hydrolysis reactions. The 4-(4-phenylbutoxy)benzoic acid is prepared through esterification and hydrolysis, the synthesis route has the characteristics of low cost, mild reaction conditions, low toxicity, convenient operation, high yield, and suitability for the industrial production.
Owner:PINGHU UCON PHARMA R&D
Spray-dried granules containing pranlukast and processes for the preparation thereof
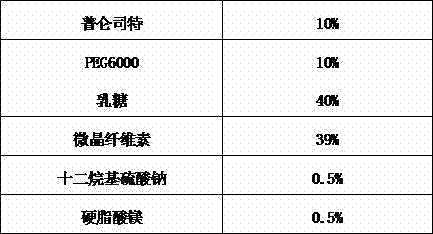
The invention provides a spray-dried granule comprising pranlukast, a water-soluble polymer and a surfactant, and a method for producing the granule. The average particle size of the pranlukast in the granule is 0.5-20 um. Thereby, not only adhesion cohesiveness and solubility of the pranlukast but also elution rate and bioavailability thereof can be improved even without coating the pranlukast with saccharides such as lactose. As a result, the dose can remarkably be reduced from that of conventional formulations. Furthermore, since the formulation can be produced without using an organic solvent, the formulation is suitable for mass-production on an industrial scale.
Owner:YUHAN
Preventive and/or Therapeutic Agents for Meniere's Disease
InactiveUS20070249695A1Effective preventionEffective therapeutic agentBiocideSenses disorderInner Ear DiseasesMedicine
The present invention relates to preventive and / or therapeutic agents for Meniere's disease, which comprise a leukotriene antagonist (such as pranlukast hydrate) as an active ingredient. Leukotriene antagonists (such as pranlukast hydrate) are effective in ameliorating various symptoms, such as hearing impairment, tinnitus, a feeling of fullness in the ear and vertigo, thus being useful as a preventive and / or therapeutic agent for Meniere's disease.
Owner:ONO PHARMA CO LTD +1
Preparation method of Pranlukast intermediate
InactiveCN101781288AReduce usageThe reaction process is simpleOrganic chemistryState of artSynthesis methods
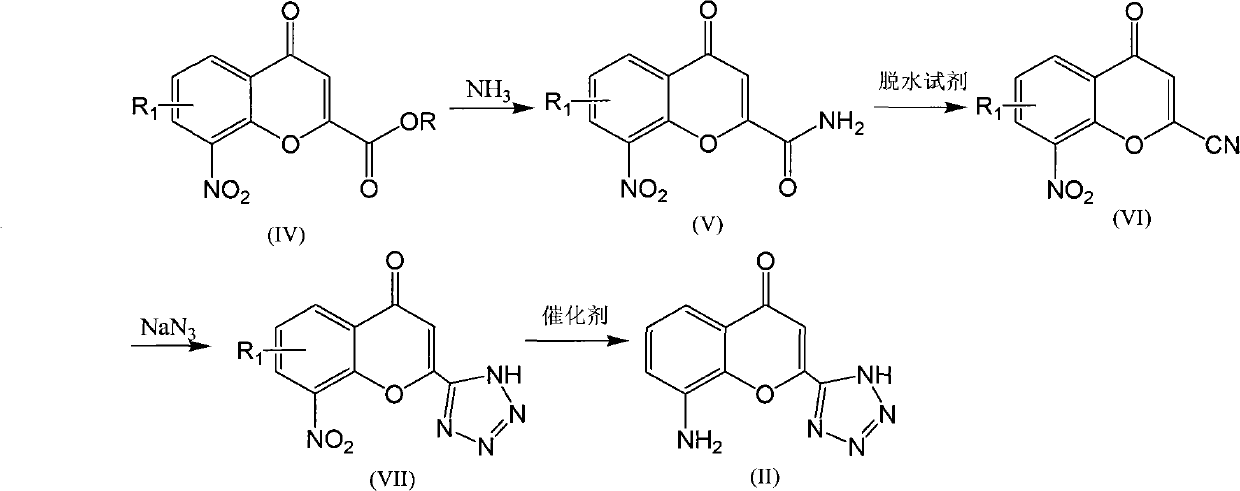
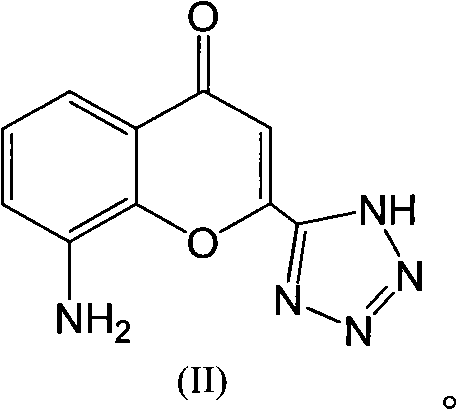
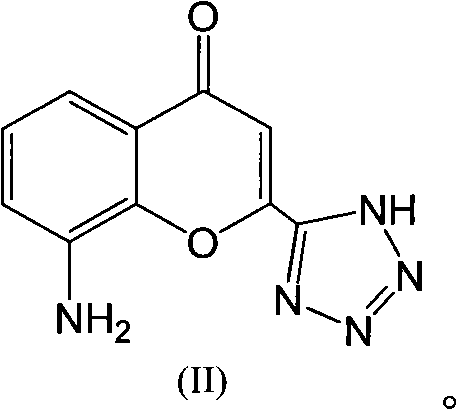
The invention discloses a preparation method of Pranlukast intermediate with the structure as shown in the formula (II), the synthesis route of which is shown as follow. Compared with the prior art, the synthesis route couples the dehalogenate and the nitro group reduction steps, so the reaction process is simplified, the reaction condition is moderate, the post treatment is relatively simple, the reaction time is shortened, and the reaction yield is improved. The invention provides a novel practical synthesis method for synthesizing Pranlukast.
Owner:ZHEJIANG UNIV
Preparation method of high-purity small-particle-size pranlukast
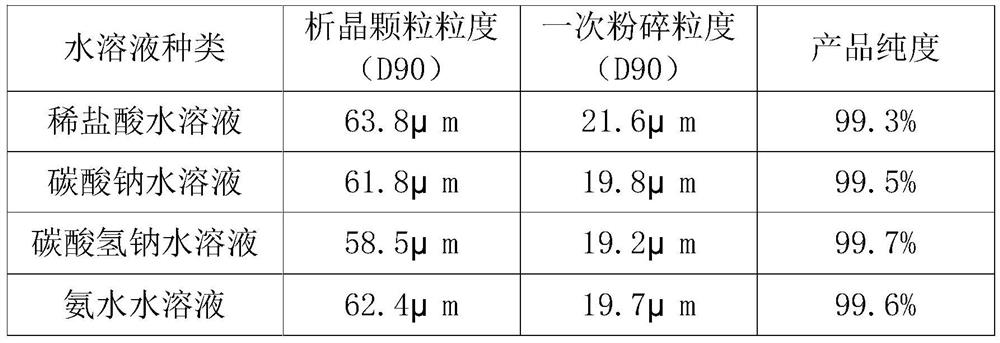
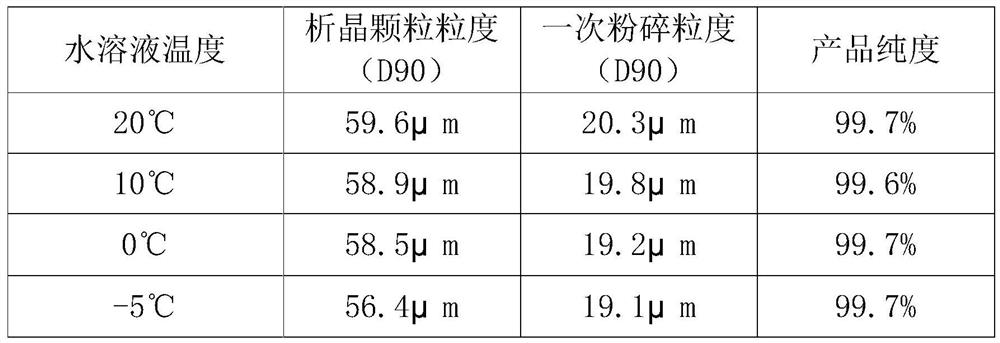
The invention provides a preparation method of high-purity small-particle-size pranlukast. The method comprises the following steps: 1, mixing and dissolving a water-insoluble organic solvent and dimethylformamide, adding a pranlukast crude product, and heating and refluxing to obtain a solution A; 2, quickly adding the solution A into an aqueous solution, and separating out a solid under quick stirring; 3, filtering, separating, drying and sieving to obtain pranlukast with the particle size distribution smaller than 60 microns; and 4, performing primary air jet pulverization on the pranlukast particles with the particle size distribution smaller than 60 microns to obtain pranlukast particles with the particle size distribution D90 smaller than or equal to 20 microns. According to the invention, the pranlukast product with the purity and the particle size meeting the requirements of raw material medicines is prepared at a time, the method is easy to operate, the crystallization process is fast, and the obtained product is easy to smash and suitable for industrial production.
Owner:HUNAN FURUI BIOPHARMA TECH CO LTD
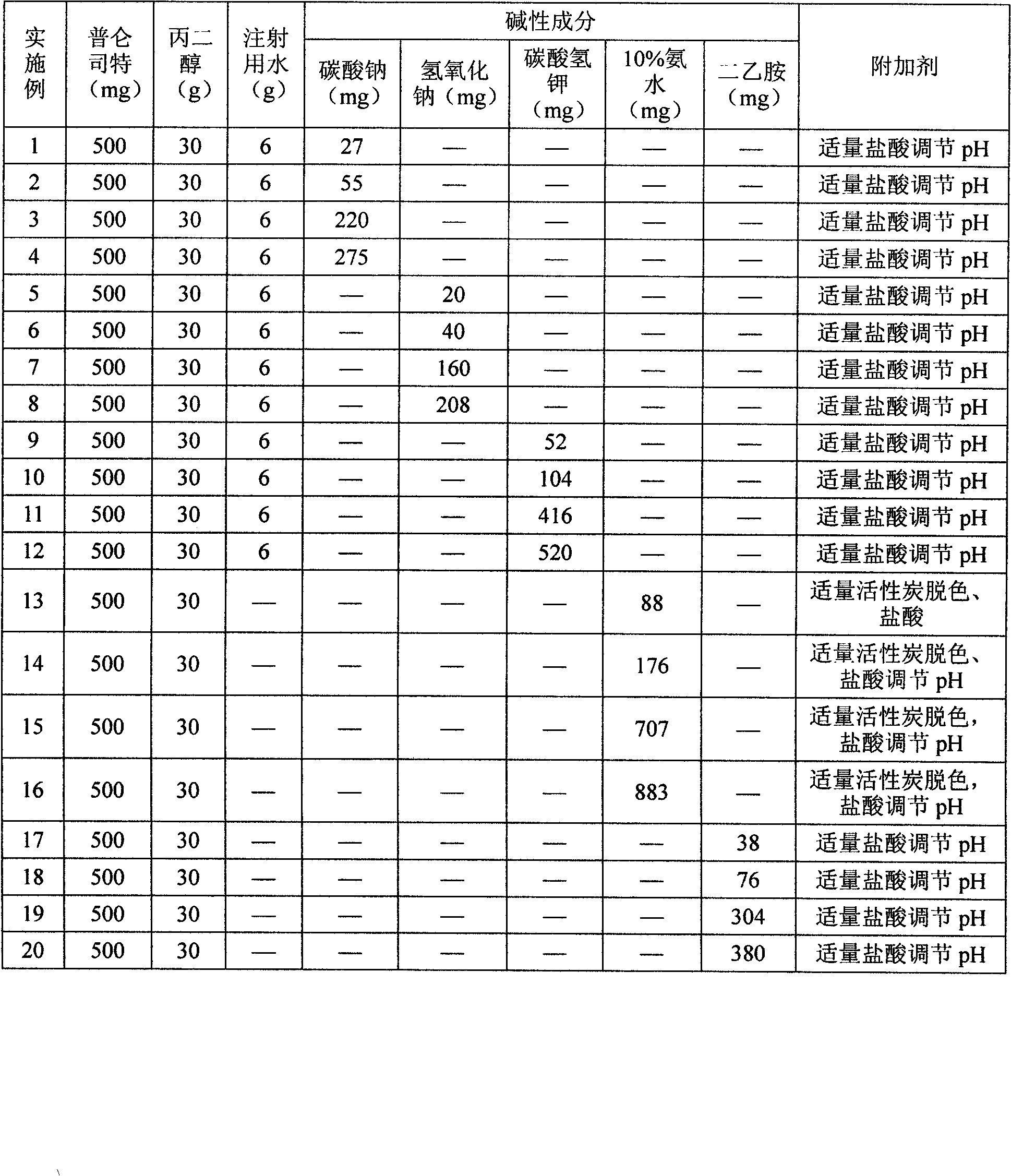
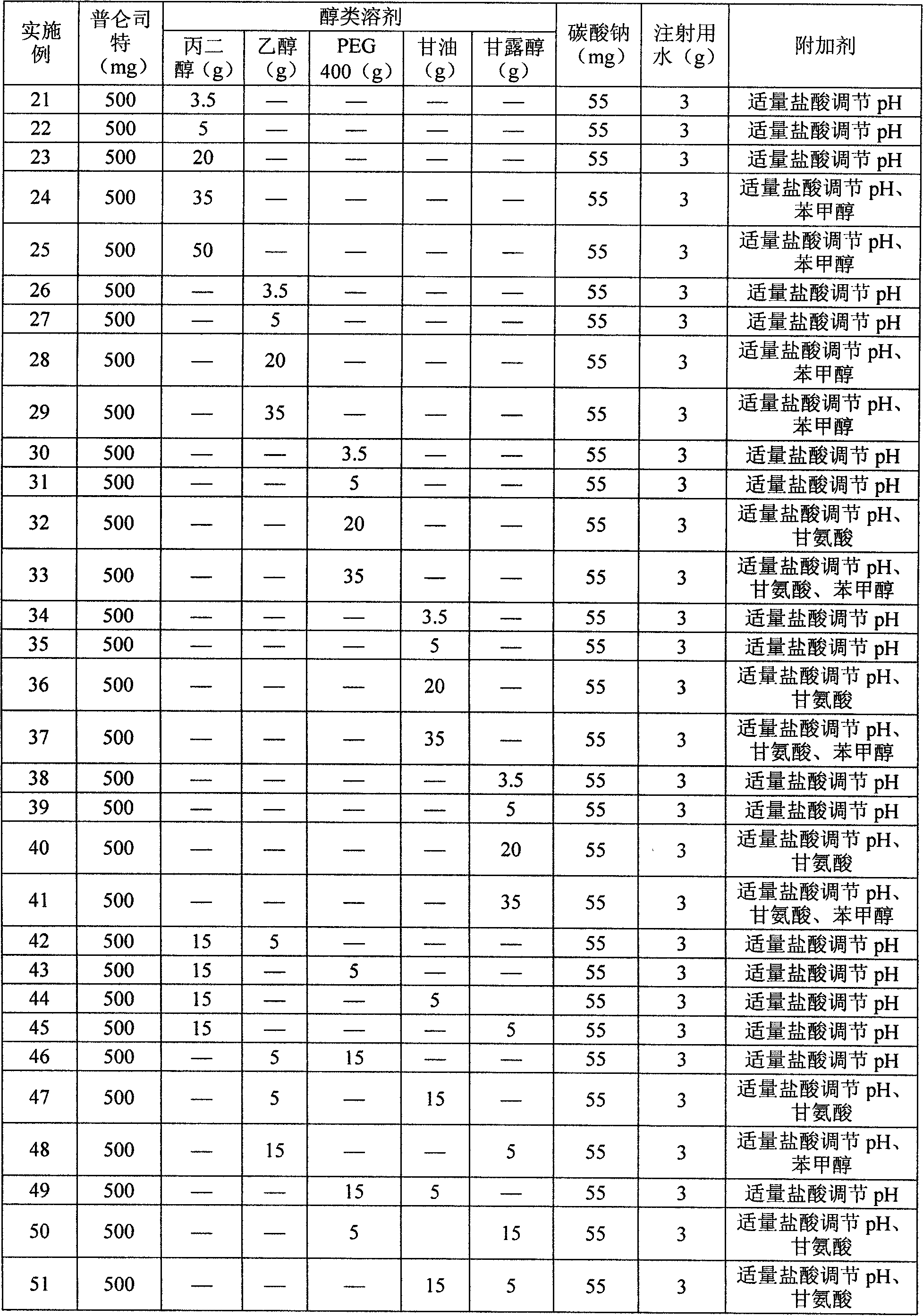
Pranlukast injection preparation
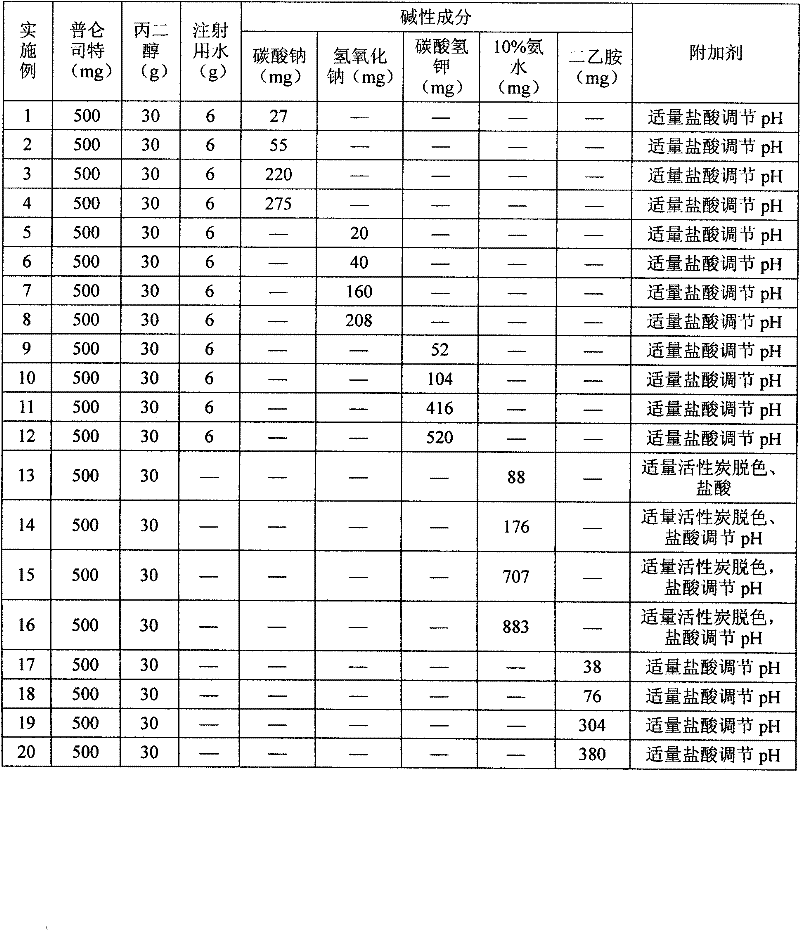
InactiveCN102178647AOvercoming the problem of low bioavailabilityImprove solubilitySenses disorderInorganic non-active ingredientsSolubilityAlcohol
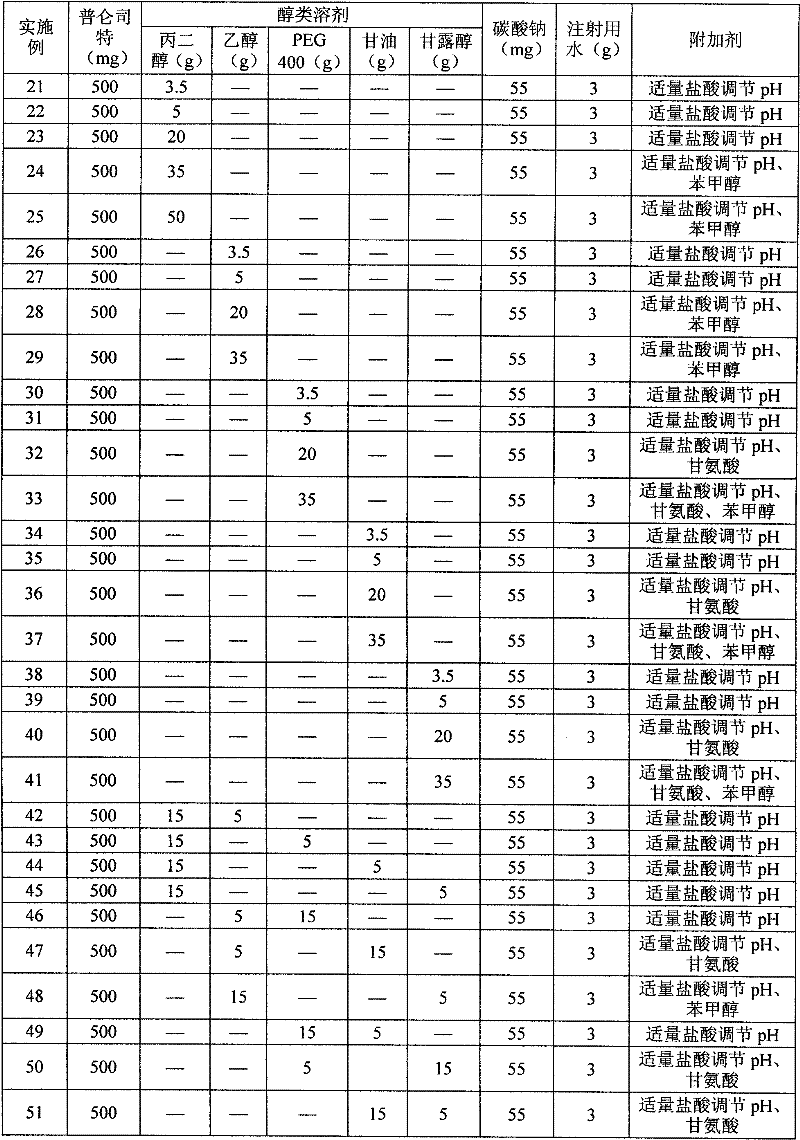
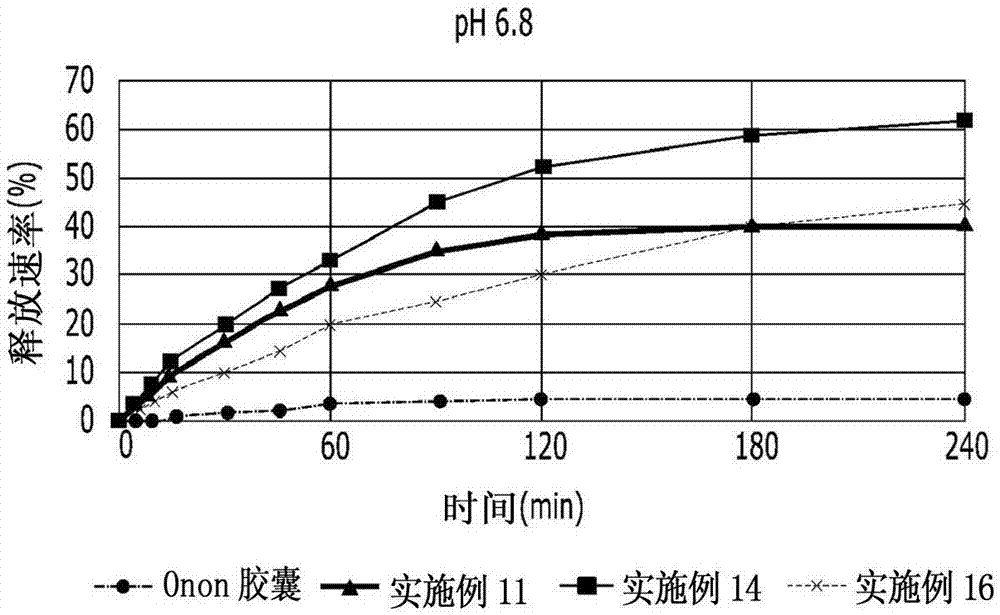
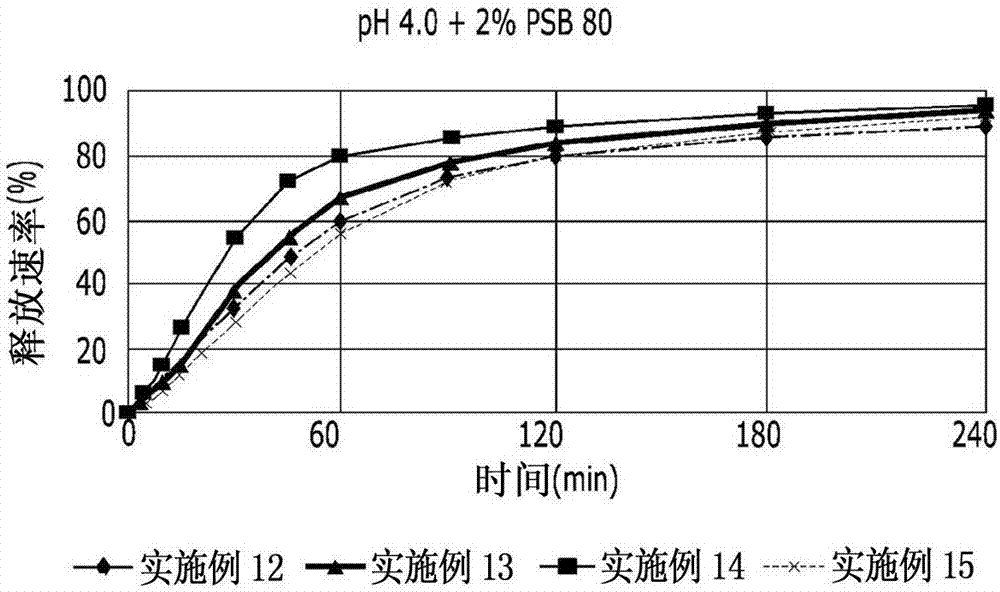
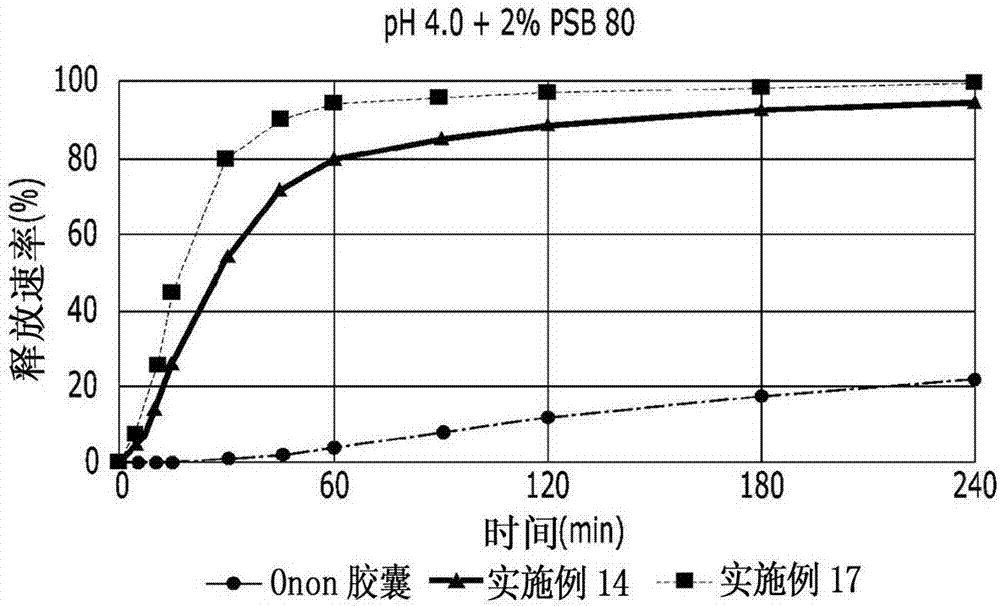
The invention relates to a pranlukast injection preparation. In the invention, an alcohols solvent which can be accepted in drugs contains effective doses of pranlukast and alkaline constituents allowed to be used in the needed drugs which can fully convert the pranlukast into water soluble salt, wherein the weight ratio of the pranlukast to the alcohols solvent is 1: (7-70) and the pH (potential of Hydrogen) value of the injection preparation is 3-10. By utilizing the injection preparation, the problems that the pranlukast has low solubility and the bioavailability of a traditional oral preparation is low are effectively and thoroughly solved; the concentration of the pranlukast in the injection preparation can reach 1%; and therefore, the drug dosage can be greatly decreased, the preparation is convenient to prepare and the drug cost is reduced.
Owner:CHONGQING HUIZHI PHARMA RES INST +2
Novel method for preparing high-purity asthma treatment drug pranlukast
ActiveCN106854202AAct as an acid-binding agentSimple processOrganic chemistryBenzopyranOrganic synthesis
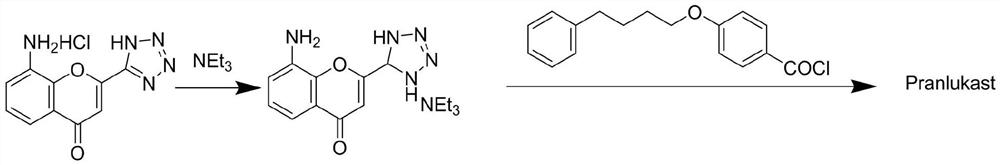
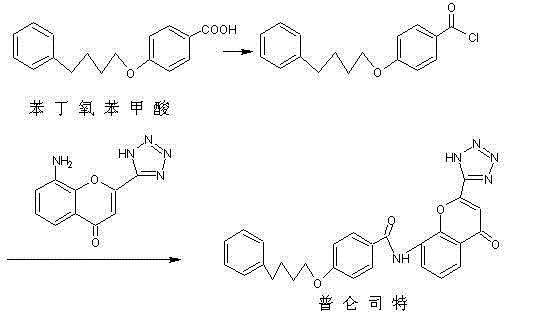
The invention discloses a novel method for preparing a high-purity asthma treatment drug pranlukast, and relates to the technical field of organic synthesis of drugs. The method comprises the following steps: carrying out neutralization and a salt forming reaction on a reaction raw material 8-amino-4-oxo-2-tetrazole-5-yl-4H-1-benzopyran hydrochloride and an alkaline substance to generate an intermediate, and carrying out an amidation reaction on the obtained intermediate and p-phenylbutoxybenzoyl chloride in a non-polar solvent to prepare the pranlukast. The whole process of the method is simple and clean, is easy to control, allows the purity of the obtained crude product to reach 99% or above, and allows the purity of a one-time purified product to reach 99.9% or more.
Owner:ANHUI HERYI CHEM
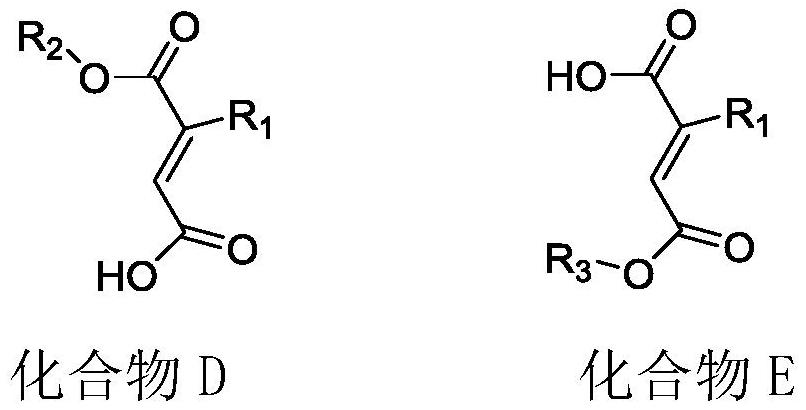
Preparation method of pranlukast intermediate
The invention provides a preparation method of a pranlukast intermediate. A compound C serving as a raw material reacts obtain the pranlukast intermediate. The preparation method has the advantages that the reaction condition is relatively mild, the operation is convenient, the purity and the yield of the obtained pranlukast intermediate are high, and the method is suitable for industrial production, so that a more valuable synthesis route is provided for preparation of pranlukast, good social benefits and economic benefits can be generated, and the economic potential is great.
Owner:HUNAN FURUI BIOPHARMA TECH CO LTD
Method for synthesizing drug pranlukast from tetrahydrofuran path
The invention discloses a new method for synthesizing pranlukast, in particular to a method for synthesizing medicinal pranlukast from tetrahydrofuran. The method takes the tetrahydrofuran as raw materials to prepare the pranlukast through ring opening, Friedel-Crafts alkylation, bromination, condensation and ring closing reactions. The method has the characteristics of readily available raw materials, simple reaction for preparation of an intermediate, high yield, safe reactions and suitability for industrial production, and can be used to prepare the medicinal pranlukast for treating asthmaand allergic rhinitis.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Antitumor agent
Owner:TOCHIGI INST OF CLINICAL PATHOLOGY
Spray-dried granules containing pranlukast and processes for the preparation thereof
The invention provides a spray-dried granule comprising pranlukast, a water-soluble polymer and a surfactant, and a method for producing the granule. The average particle size of the pranlukast in thegranule is 0.5-20 um. Thereby, not only adhesion cohesiveness and solubility of the pranlukast but also elution rate and bioavailability thereof can be improved even without coating the pranlukast with saccharides such as lactose. As a result, the dose can remarkably be reduced from that of conventional formulations. Furthermore, since the formulation can be produced without using an organic solvent, the formulation is suitable for mass-production on an industrial scale.
Owner:YUHAN
Preparation method of Pranlukast intermediate
InactiveCN101781288BReduce usageThe reaction process is simpleOrganic chemistrySynthesis methodsCombinatorial chemistry
The invention discloses a preparation method of Pranlukast intermediate with the structure as shown in the formula (II), the synthesis route of which is shown as follow. Compared with the prior art, the synthesis route couples the dehalogenate and the nitro group reduction steps, so the reaction process is simplified, the reaction condition is moderate, the post treatment is relatively simple, the reaction time is shortened, and the reaction yield is improved. The invention provides a novel practical synthesis method for synthesizing Pranlukast.
Owner:ZHEJIANG UNIV
A new method for preparing high-purity asthma medicine pranlukast
ActiveCN106854202BAct as an acid-binding agentSimple processOrganic chemistryTetrazoleOrganic synthesis
Owner:ANHUI HERYI CHEM
A kind of preparation method of pranlukast
The invention belongs to the field of medicines and especially relates to a preparation method for pranlukast. The method comprises the following steps: A) adding 4-bromobenzoic acid and thionyl chloride into a solvent and reacting, thereby acquiring 4-bromo-benzoyl chloride; B) adding the 4-bromo-benzoyl chloride acquired in the step A), alkali and 3-amino-2-ethyl iodobenzoate into the solvent and reacting, thereby acquiring 2-iodine-3-(4-bromobenzamide) ethyl benzoate; C) adding the 2-iodine-3-(4-bromobenzamide) ethyl benzoate acquired in the step B), alkali and 1-(1H-tetrazole-5-group) aceton into the solvent and reacting, thereby acquiring 4-bromine-N-(4-oxo-2-(1H-tetrazole-5-group)-4H-benzopyrone-8-group) benzamide; D) adding the 4-bromine-N-(4-oxo-2-(1H-tetrazole-5-group)-4H-benzopyrone-8-group) benzamide acquired in the step C), catalyst and alkali into the solvent and reacting, thereby acquiring the product. The method provided by the invention is simple and practicable in process, low in production cost and high in yield.
Owner:烟台万润药业有限公司
Antitumor agent
Pharmaceuticals which are effective for treatment, prevention, and the like of cancer and have less side effects are disclosed. The antitumor agent of the present invention comprises as an effective ingredient at least one leukotriene inhibitor. Examples of the leukotriene inhibitor includes leukotriene production inhibitors and leukotriene receptor antagonists, and preferred specific examples of the leukotriene inhibitor include montelukast, zafirlukast, pranlukast, and zileuton; pharmaceutically acceptable salts of these compounds; and pharmaceutically acceptable solvates of these compounds and the salts. The leukotriene inhibitor can also be used as a relieving agent for pain accompanying a tumor(s), and as a stromal hyperplasia inhibitor.
Owner:TOCHIGI INST OF CLINICAL PATHOLOGY
Antitumor agent
Pharmaceuticals which are effective for treatment, prevention, and the like of cancer and have less side effects are disclosed. The antitumor agent of the present invention comprises as an effective ingredient at least one leukotriene inhibitor. Examples of the leukotriene inhibitor includes leukotriene production inhibitors and leukotriene receptor antagonists, and preferred specific examples of the leukotriene inhibitor include montelukast, zafirlukast, pranlukast, and zileuton; pharmaceutically acceptable salts of these compounds; and pharmaceutically acceptable solvates of these compounds and the salts. The leukotriene inhibitor can also be used as a relieving agent for pain accompanying a tumor(s), and as a stromal hyperplasia inhibitor.
Owner:TOCHIGI INST OF CLINICAL PATHOLOGY
P-phenylbutoxy benzoic acid and preparation method thereof
InactiveCN105732367ALow priceImprove reaction efficiencyOxygen-containing compound preparationOrganic compound preparationBenzeneBenzoic acid
The invention discloses a preparation method of p-phenylbutoxy benzoic acid. The preparation method comprises the following steps: by using 1,4-dibromobutane as a raw material, carrying out reaction on the 1,4-dibromobutane and benzene to obtain 4-phenylbromobutane; carrying out reaction on the 4-phenylbromobutane and methyl p-hydroxybenzoate to obtain methyl p-phenylbutoxy benzoate; and oxidizing the methyl p-phenylbutoxy benzoate, and meanwhile, carrying out hydrolysis to obtain the pranlukast intermediate p-phenylbutoxy benzoic acid. The p-phenylbutoxy benzoic acid prepared by the method has the advantages of high purity and high yield; and the method has the advantages of cheap and accessible raw materials, mild preparation conditions, high controllability and low production cost.
Owner:叶芳
Pranlukast solid dispersion and preparation method thereof
InactiveCN103751108AThe preparation process is stableGood reproducibilityPowder deliveryRespiratory disorderActive agentDrugs preparations
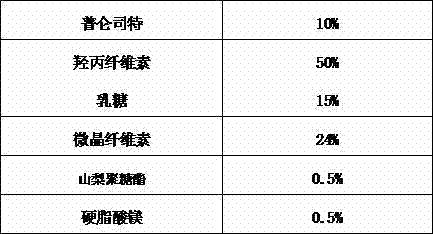
The invention relates to pranlukast solid dispersion and a preparation method thereof, belonging to the field of pharmaceutical preparations. Particularly, the invention provides solid dispersion and a pharmaceutical composition. The solid dispersion is composed of pranlukast, a water-soluble polymer and a surface active agent; and the pharmaceutical composition is composed of pharmaceutically acceptable carriers. The solid dispersion disclosed by the invention is capable of increasing solubility of medicines, improving viscidity of pranlukast and prompting absorption, and therefore, dissolution rates and bioavailability of medicines are increased.
Owner:BEIJING VENTUREPHARM BIOTECH
Pranlukast injection preparation
InactiveCN102178647BOvercoming the problem of low bioavailabilityImprove solubilitySenses disorderInorganic non-active ingredientsSolubilityHydrogen
The invention relates to a pranlukast injection preparation. In the invention, an alcohols solvent which can be accepted in drugs contains effective doses of pranlukast and alkaline constituents allowed to be used in the needed drugs which can fully convert the pranlukast into water soluble salt, wherein the weight ratio of the pranlukast to the alcohols solvent is 1: (7-70) and the pH (potentialof Hydrogen) value of the injection preparation is 3-10. By utilizing the injection preparation, the problems that the pranlukast has low solubility and the bioavailability of a traditional oral preparation is low are effectively and thoroughly solved; the concentration of the pranlukast in the injection preparation can reach 1%; and therefore, the drug dosage can be greatly decreased, the preparation is convenient to prepare and the drug cost is reduced.
Owner:CHONGQING HUIZHI PHARMA RES INST +2
Composition of pranlukast-containing solid preparation with improved bioavailability and method for preparing same
InactiveCN108012526AIncreased release rateImprove bioavailabilityPill deliveryPharmaceutical non-active ingredientsBioavailabilityPranlukast
The present invention relates to a pharmaceutical composition containing pranlukast and a method for preparing the same. The present invention has a significantly improved pranlukast release rate andbioavailability compared with conventional pranlukast-containing products.
Owner:SAM A PHARMA
A kind of refining purification method of pranlukast intermediate phenbutoxybenzoic acid
ActiveCN104177252BQuality improvementLow priceOrganic compound preparationCarboxylic compound separation/purificationBenzoic acidOrganic base
A method for refining and purifying pranlukast intermediate phenbutoxybenzoic acid, the method comprising the following steps: (1) Salt-forming reaction: adding an appropriate amount of protic or aprotic phenbutoxybenzoic acid crude product (substrate) Add a certain amount of aqueous or alcoholic solution of inorganic base or organic base and stir to fully form a salt with phenylbutoxybenzoic acid; (2) Post-process the salt-forming solution to precipitate High-purity phenbutoxybenzoic acid crystals: filter out the impurity-dicarboxylate solids precipitated in the salt-forming solution, distill and concentrate the filtrate, add an appropriate amount of water to the concentrate to form a homogeneous aqueous solution, and then acidify to precipitate crystals. The high-purity phenbutoxybenzoic acid product is obtained by filtration, which has high purity and high yield.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
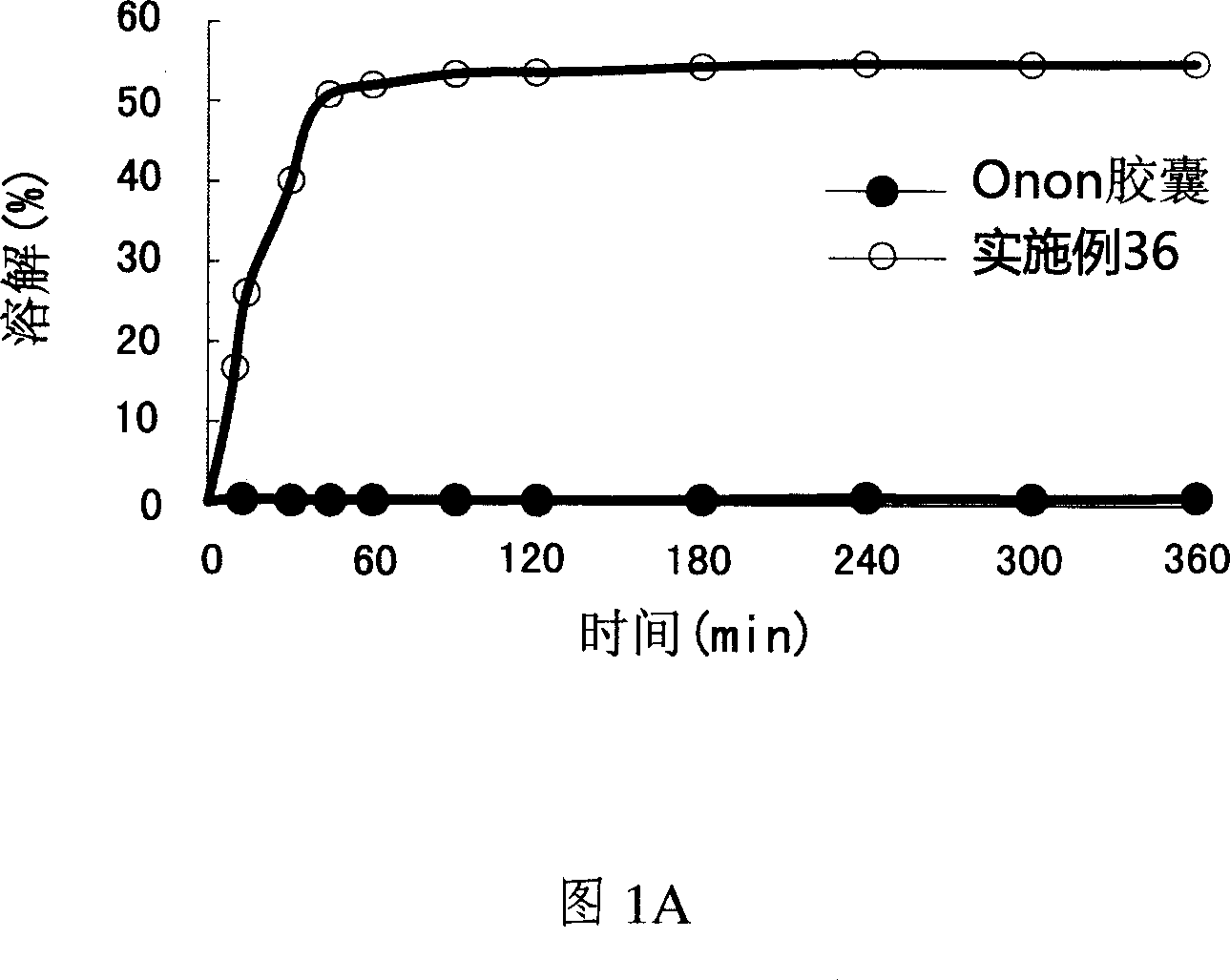
Pharmaceutical composition of pranlukast solid-dispersion with improved initial dissolution rate and the method of preparing the same
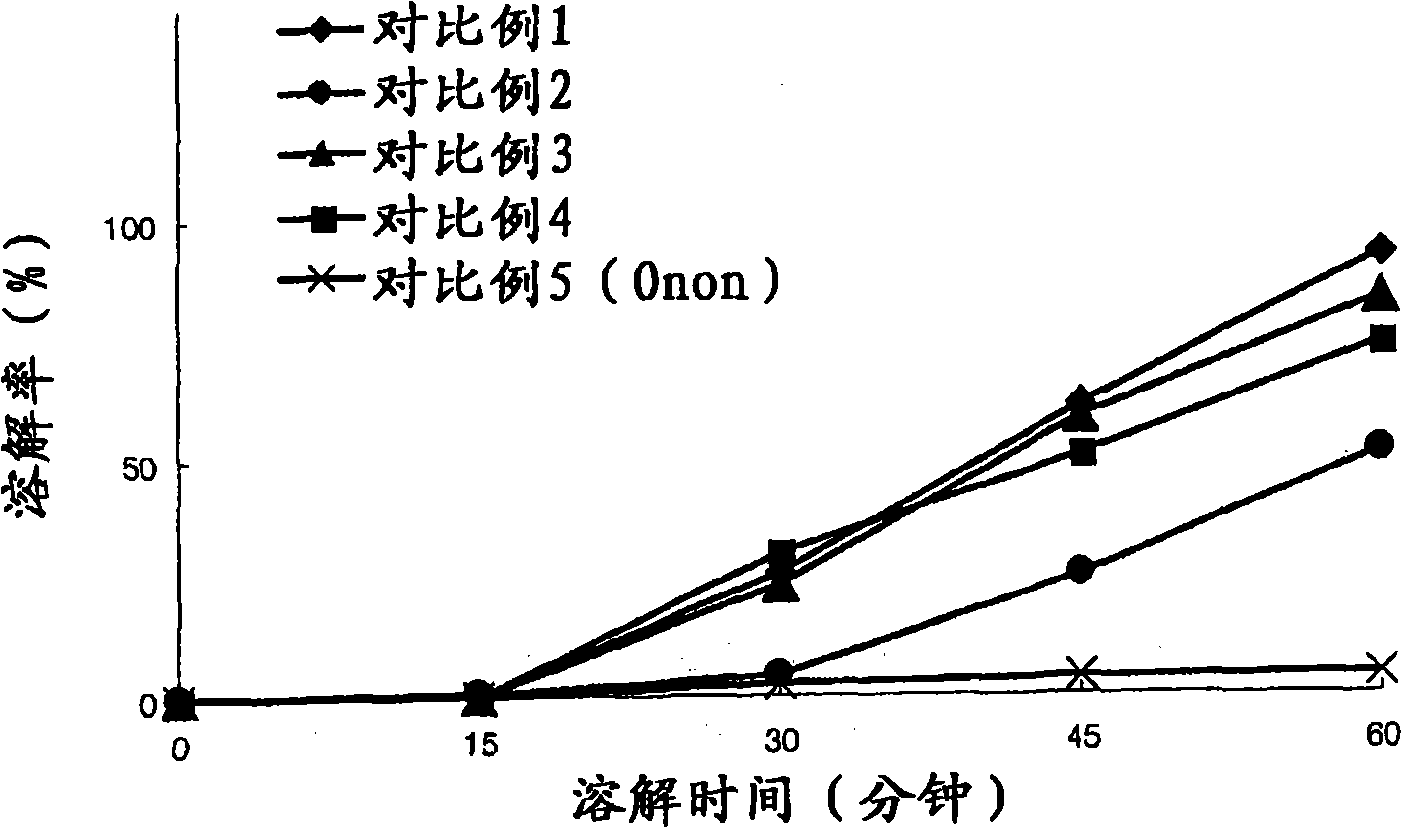
ActiveCN101282716AImproved initial dissolution propertiesImprove bioavailability in vivoPowder deliverySenses disorderMedicineDissolution
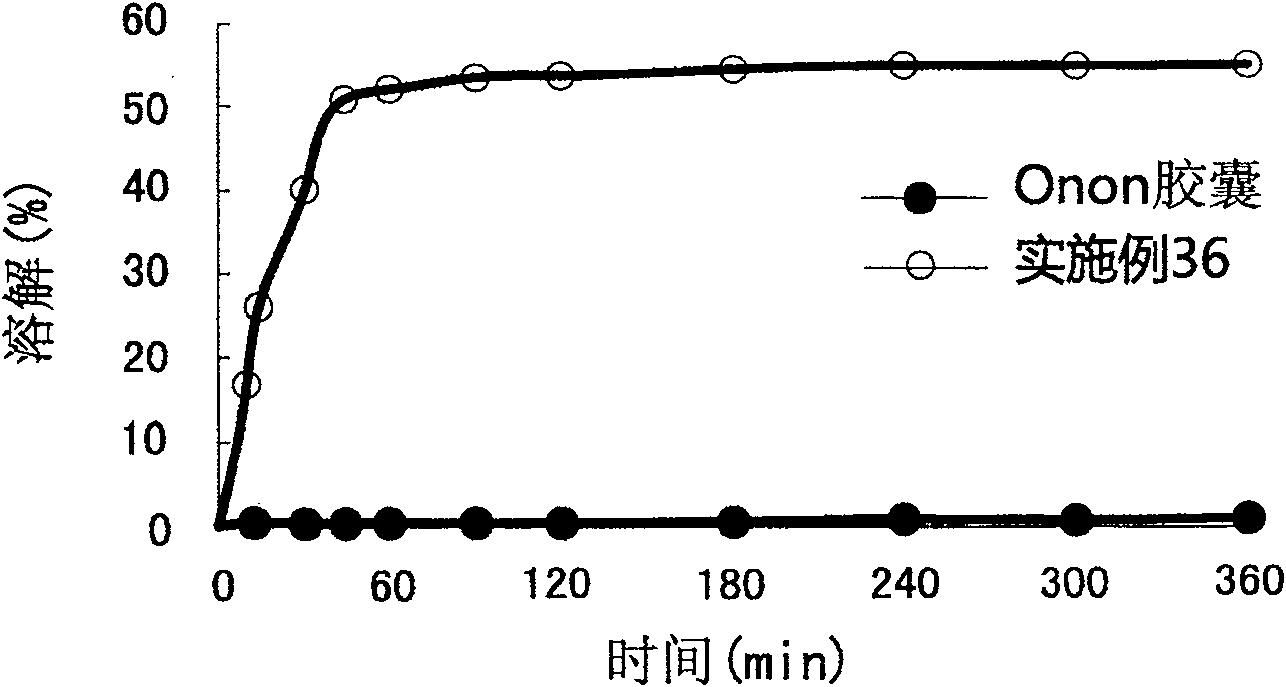
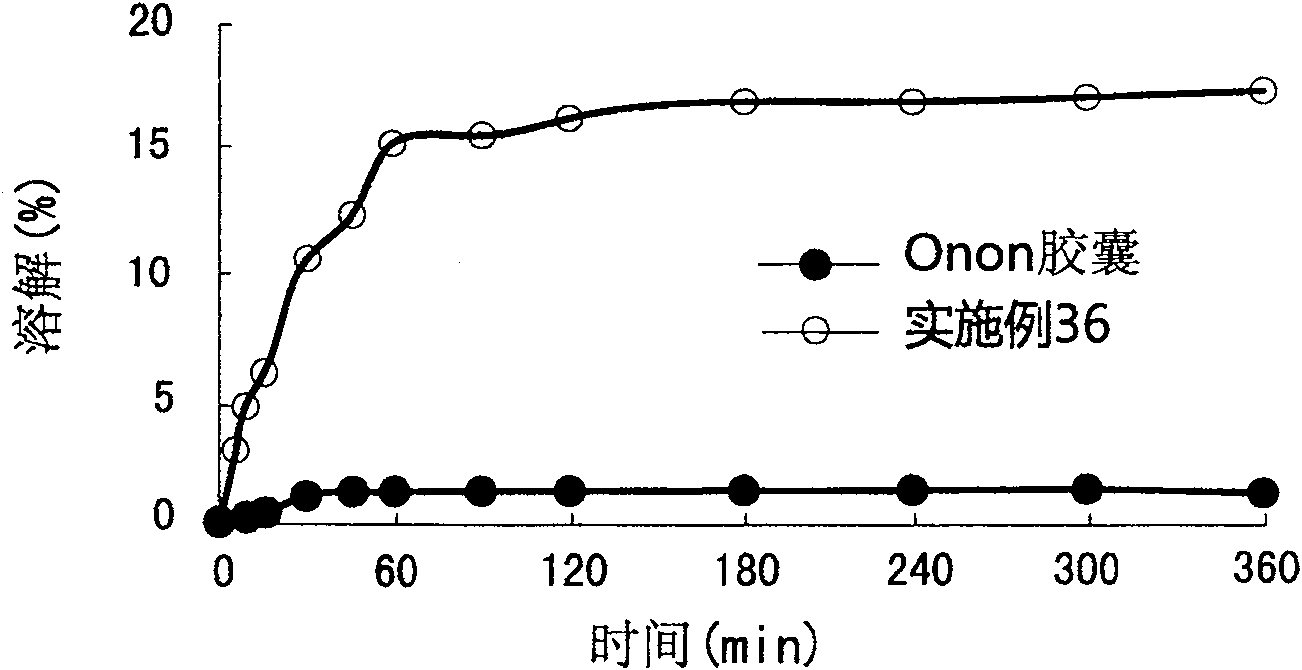
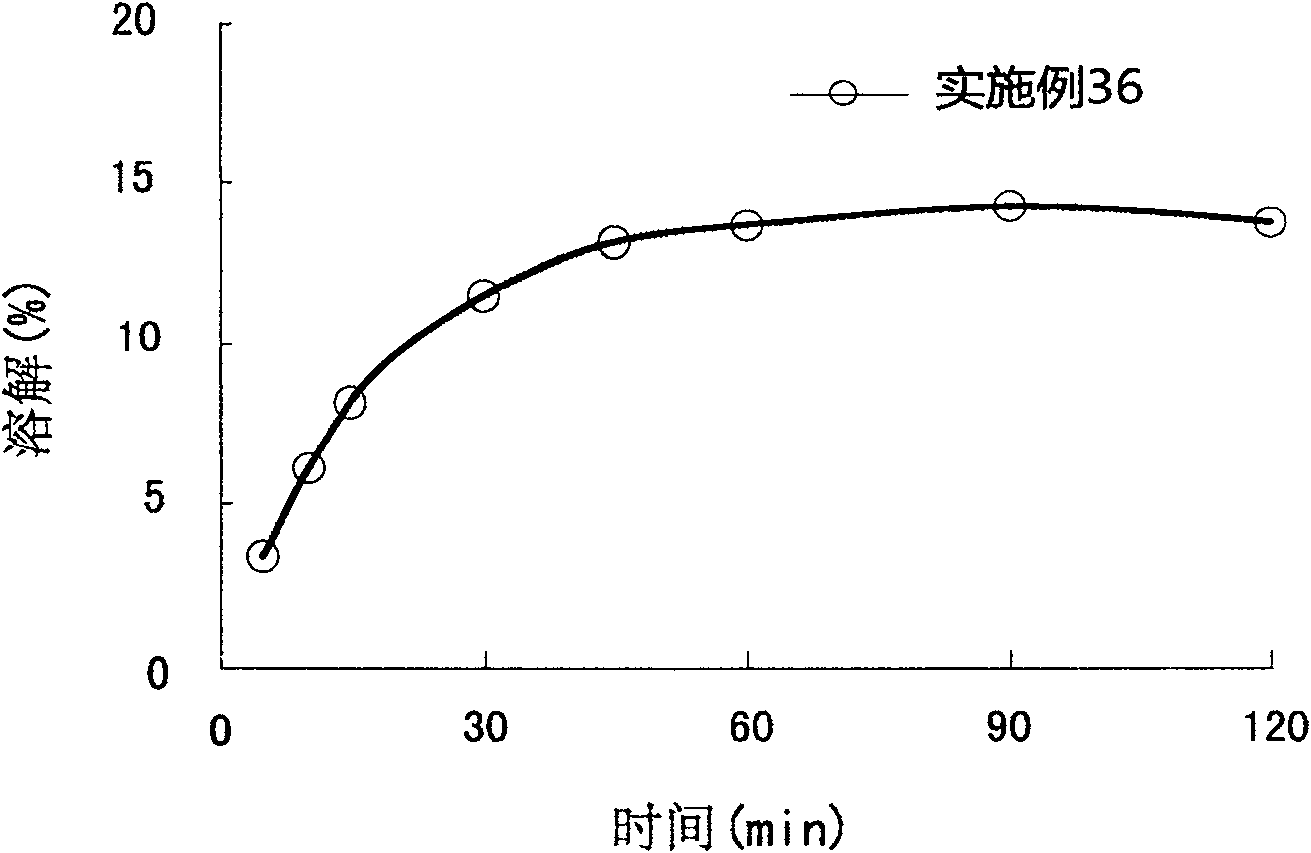
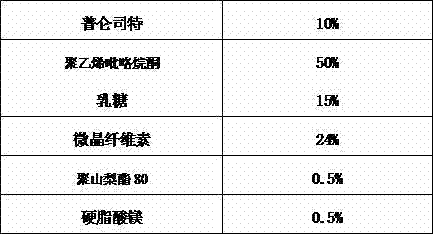
The present invention relates to a pharmaceutical composition of pranlukast solid-dispersion with an improved initial dissolution rate and the preparation method thereof. More particularly, it relates to a pharmaceutical composition of pranlukast solid-dispersion prepared by mixing pranlukast solid-dispersion and anticoagulation agent with a certain range of HLB at a elevated temperature, which can be further granulated and capsulated, thus enabling to improve initial dissolution rate of pranlukast by solving the serious problem of pranlukast solid-dispersion to stick to capsule walls and, to improve bioavailability because it shows superior Cmax and AUC based on the same administration dose, as comared to the commercial pharmaceutical composition of pranlukast formulated by conventional method, along with the preparation method thereof.
Owner:SK CHEM CO LTD
Preparation method of pranlukast intermediate
InactiveCN111960957AReduce pollutionImprove pollutionOrganic chemistryOrganic compound preparationBenzoic acidPhenacyl
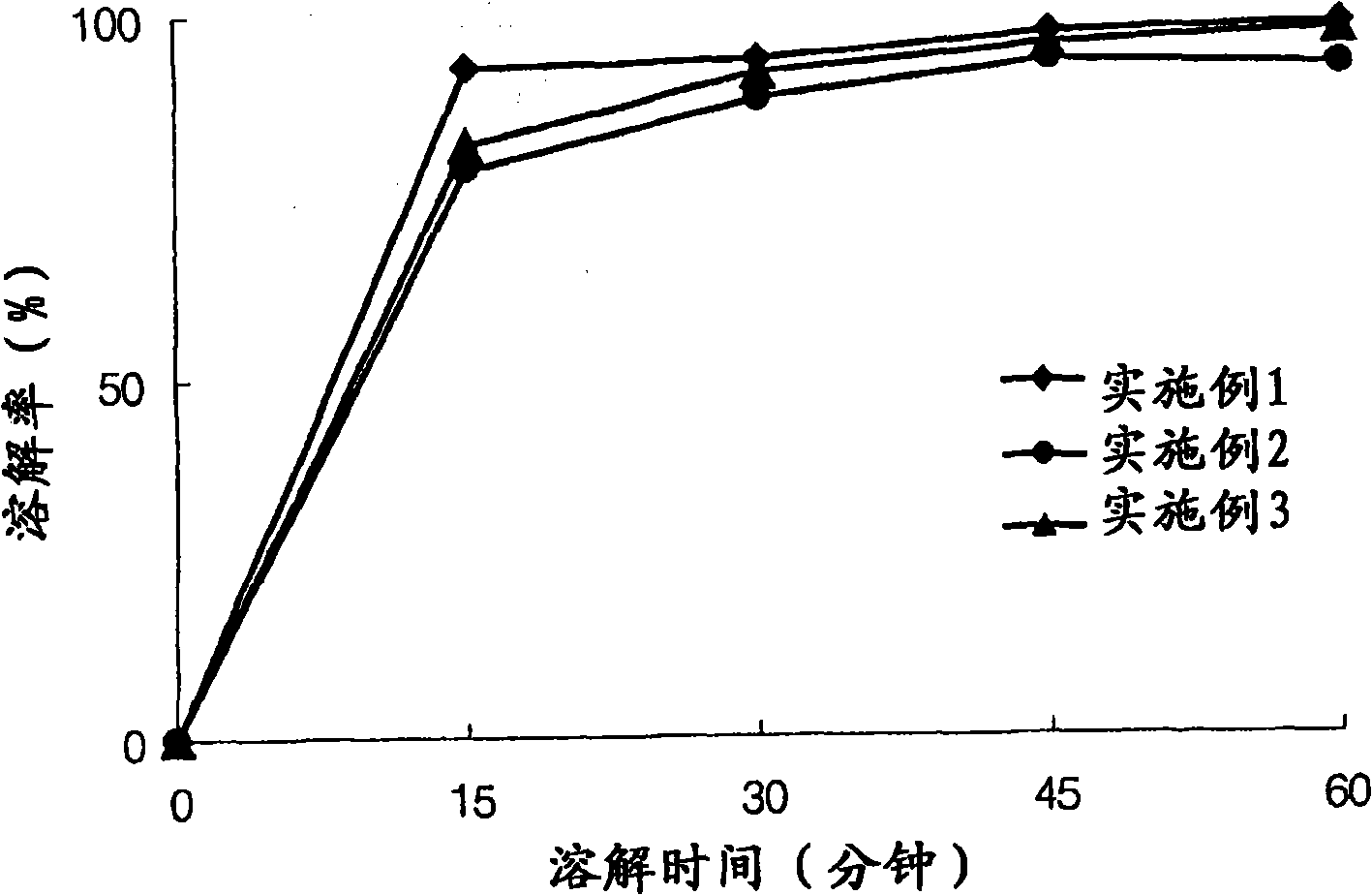
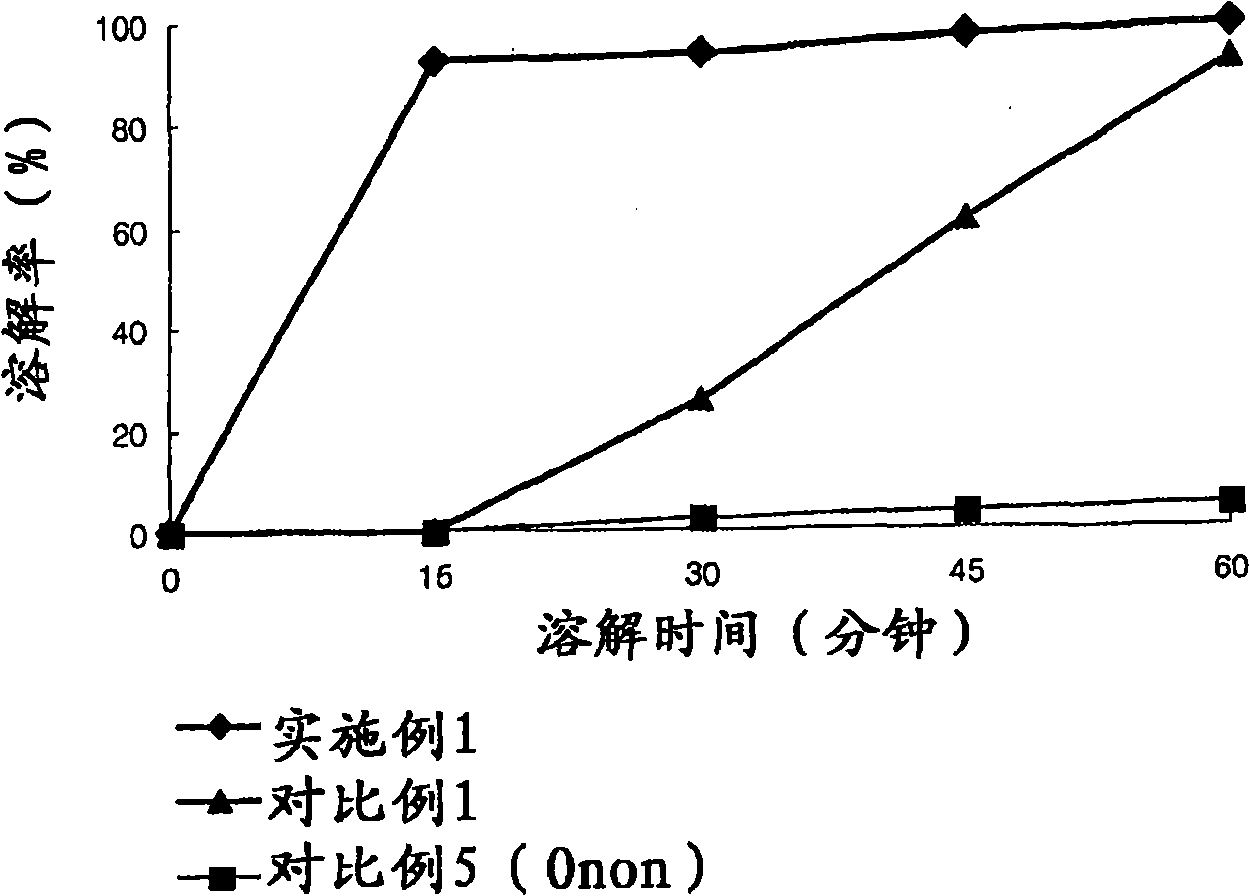
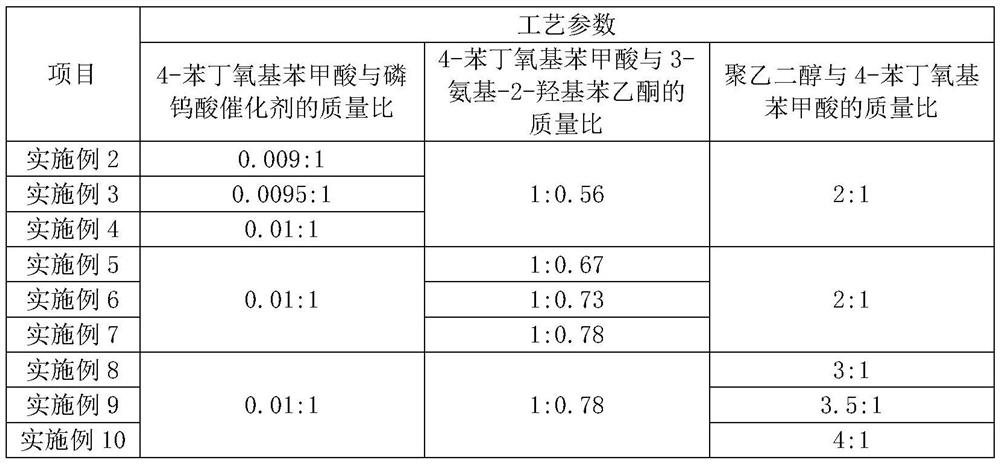
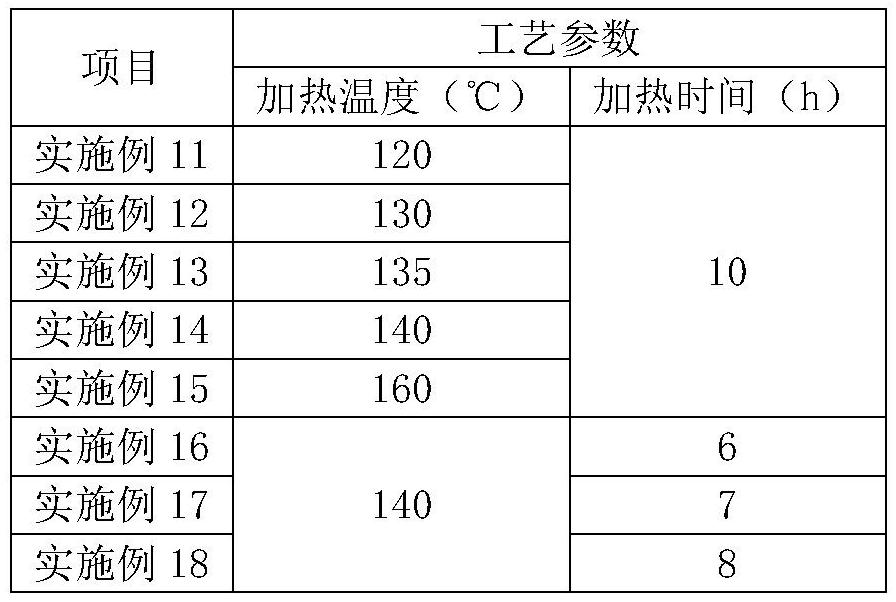
The invention relates to the field of medicine synthesis, and particularly discloses a preparation method of a pranlukast intermediate. The preparation method of the pranlukast intermediate comprisesthe following steps: taking 4-phenylbutoxy benzoic acid and 3-amino-2-hydroxyacetophenone as raw materials, adding a phosphotungstic acid catalyst into a reaction system, and carrying out amidation reaction to obtain a target product 3-[4-(4-phenylbutoxy)benzoylamino]-2-hydroxyacetophenone. The preparation method provided by the invention has the advantage of environmental protection on the premise of ensuring the yield and purity of the target product.
Owner:太仓康源化建医药有限公司
Preparation method for pranlukast
InactiveCN108912100AReduce energy consumptionReduce manufacturing costOrganic chemistrySolubilityN dimethylformamide
The invention provides a preparation method for pranlukast. According to the preparation method, N,N-dimethylformamide, dimethylacetamide or N-methylpyrrolidone is used as a reaction solvent for a Claisen condensation reaction; after the reaction is completed, a condensation reaction solution and an acid solution are directly mixed for a cyclization reaction; the cyclization is carried out in a mixed system of a polar organic solvent (N,N-dimethylformamide, dimethylacetamide or N-methylpyrrolidone) and water; and the mixed system has good product solubility and is favorable for the implementation of the cyclization reaction. The preparation method provided by the invention can carry out a next-step reaction without separating an excess compound, is a one-step method for synthesizing pranlukast, is simple to operation and can improve product yield. Furthermore, the preparation method provided by the invention has a cyclization reaction temperature of only 0 to 40 DEG C, so ring closurecan be achieved at a low temperature; thus, the method has low energy consumption and is reduced in preparation cost.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com