Novel method for preparing high-purity asthma treatment drug pranlukast
The technology of an asthma drug and a new method, which is applied in the field of drug organic synthesis, can solve the problems of cumbersome operation, high cost, low content of crude prankast, and achieves the effect of simple process and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
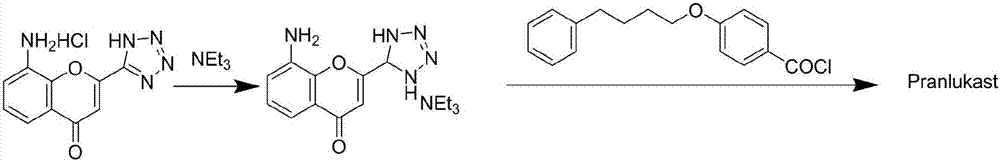
[0022] Dissolve 80g of 8-amino-4-oxo-2-tetrazol-5-yl-4H-1benzopyran hydrochloride in 500ml of dichloromethane, add 65g of triethylamine dropwise, and reflux after the dropwise reaction 2h, recover dichloromethane, stir the residue at 0°C for 30min, and filter with suction to obtain the intermediate 8-amino-4-oxo-2-pyrazol-5-yl-4H-1-benzopyran mono Triethylamine salt 93g.
[0023] 8-amino-4-oxo-2-backazol-5-yl-4H-1-benzopyran monotriethylamine salt 67g, p-phenylbutoxybenzoyl chloride 60g drop into a container containing 700ml dichloromethane In a 1000ml reaction bottle, stir and heat up to reflux for 6 hours, recover dichloromethane, add 1200ml of 5% alkaline water to the residue, stir, add a little activated carbon for decolorization, freeze and crystallize to obtain prankast sodium salt, and then dissolve the obtained prankast sodium salt Add ster sodium salt into 500ml of ethanol, add 500ml of water, adjust the pH to 6.8-7.2 with hydrochloric acid under stirring, after rete...
Embodiment 2
[0025] Dissolve 80 g of 8-amino-4-oxo-2-tetrazol-5-yl-4H-1 benzopyran hydrochloride in 500 ml of dichloromethane, add 50 g of pyridine dropwise, and reflux for 2 hours after the drop is complete. Recover dichloromethane, stir the residue at 0°C for 30 minutes, and filter with suction to obtain the intermediate 8-amino-4-oxo-2-pyrazol-5-yl-4H-1-benzopyran monopyridinium salt 86g.
[0026] Put 62g of 8-amino-4-oxo-2-backazol-5-yl-4H-1-benzopyran monopyridinium salt and 60g of p-phenylbutoxybenzoyl chloride into 1000ml of 700ml of dichloromethane for reaction In the bottle, after stirring and raising the temperature to reflux for 6 hours, reclaim dichloromethane, add 1200ml of 5% alkaline water to the residue, stir, add a little activated carbon for decolorization, freeze and crystallize to obtain prankast sodium salt, and then dissolve the obtained prankast Put the sodium salt into 500ml of ethanol, add 500ml of water, adjust the pH to 6.8-7.2 with hydrochloric acid under stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com