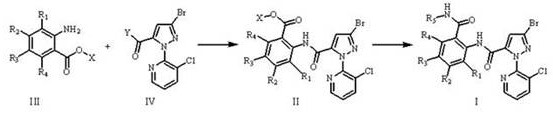
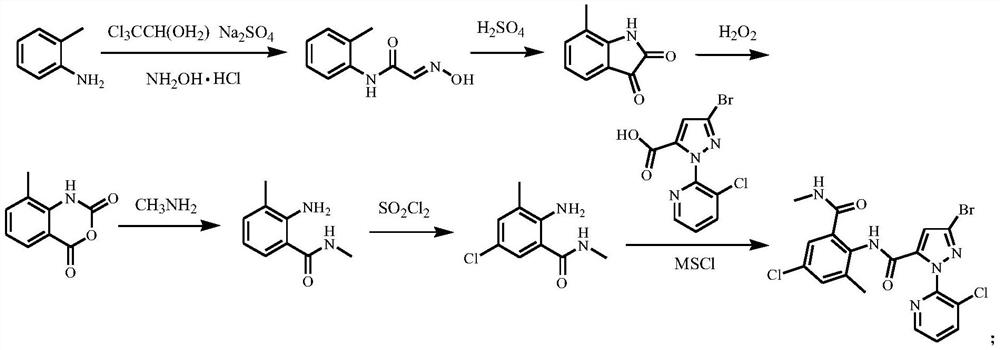
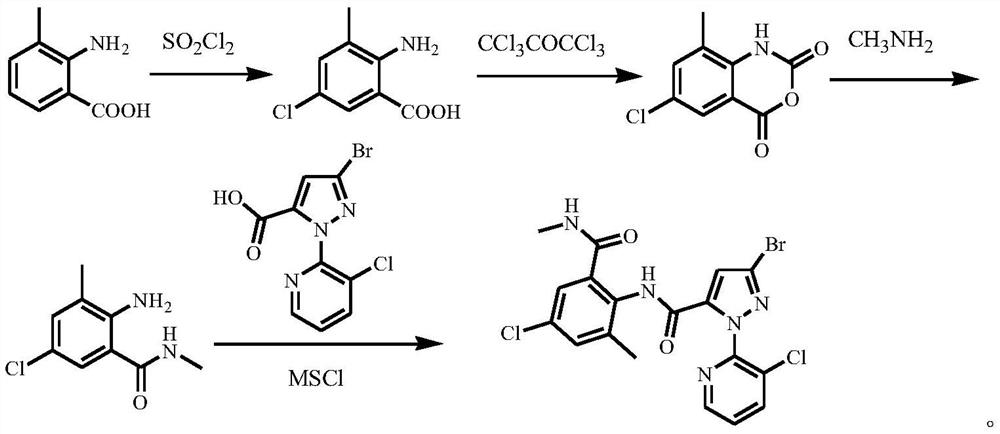
Method for preparing o-formamidobenzamide compound
An o-formamidobenzamide and compound technology, which is applied in the field of organic synthetic chemistry, can solve the problems of high solvent consumption, complicated post-processing, frequent replacement, etc., and achieves the effects of speeding up the reaction, inhibiting the generation of impurities and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 1) Synthesis of methyl 2-nitro-3-methylbenzoate:
[0064] 36.9g of 2-nitro-3-methylbenzoic acid and 110.8g of dichloroethane were added to the four-necked flask, the stirring was turned on and the temperature was raised to 50°C, and 28.6g of SOCl was slowly added dropwise. 2 , after the 0.5h dropwise addition, the temperature was raised to 65°C for 0.5h, the temperature of the raw materials was lowered to 40°C after the completion of the reaction, and 7.8g methanol was added dropwise. , add 158g methanol to dissolve for later use.
[0065] 2) Synthesis of methyl 2-amino-3-methylbenzoate:
[0066] The product solution in the step (1) was transferred to the hydrogenation kettle, 0.2 g of 10% palladium carbon was added, and the hydrogenation kettle was closed. After nitrogen replacement for 3 times, 0.5 MPa hydrogen was introduced, and the stirring was started at 60 °C for reaction for 5 h. After hot filtration, the filtrate was distilled under reduced pressure to obtain...
Embodiment 2
[0076] 1) Synthesis of methyl 2-amino-5-bromo-3-methylbenzoate:
[0077] Put 30g of methyl 2-amino-3-methylbenzoate into a four-necked flask, add 100g of 50% glacial acetic acid, and add 40g of 40% hydrobromic acid dropwise with stirring at room temperature. , slowly drip 12.5g 30%H 2 O 2 , 15min was added dropwise, kept for 0.5h until the reaction was completed, 1.5g of sodium bisulfite was added, NaOH was used to adjust the pH of the system to 5-6, cooling and suction filtration, and drying to obtain 42.8g of the product. The purity determined by HPLC was 97.9%, and the yield was 94.5%.
[0078] 2) Synthesis of methyl 2-amino-5-cyano-3-methylbenzoate:
[0079] The methyl 2-amino-5-bromo-3-methylbenzoate obtained in the previous step, 200 g of N-methylpyrrolidone and 16.2 g of cuprous cyanide were put into the reaction flask, and the temperature was raised to reflux and kept for 3 to 5 hours. After completion, 120g of 15% ammonia water and 140g of dichloroethane were adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com