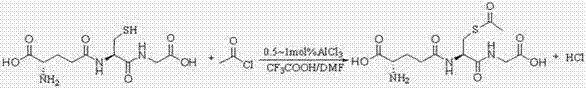
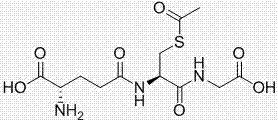
Method for catalytically synthesizing s-acetyl-l-glutathione in mixed solvent
A technology of glutathione and synthesis method, which is applied to the preparation methods of peptides, chemical instruments and methods, peptides, etc., and can solve the problems of difficulty in selective acylation of sulfhydryl groups, difficult availability of acetyl-CoA, and unsuitability for large-scale production. , to achieve the effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 50 g (0.16 mol) of glutathione, 106 mg of AlCl 3 , 200mL of trifluoroacetic acid and 200mL of DMF, stir well, then raise the temperature to 40°C, add 13.8g (0.176mol) of acetyl chloride dropwise while stirring, and take 10min. Methanol was incubated at 40°C for 15 minutes, and the solvent was recovered under reduced pressure after the reaction. Add 900mL of acetone / water (2:1) mixed solution to it and stir to dissolve. After dissolving, add triethylamine dropwise to adjust the pH to about 3. Add 300mL of acetone dropwise under slow stirring, let stand for crystallization for 1 hour after dropping, slowly cool down to 0°C, crystallize for 3 hours, filter with suction, rinse the filter cake with about 50-100mL of solvent and dry it at 70°C, that is S-acetyl-L-glutathione product 50.1g was obtained, the product yield was 89.5%, and the purity of the product detected by HPLC was greater than 99.3%.
Embodiment 2
[0024] Add 50g (0.16mol) of glutathione, 200mL of trifluoroacetic acid and 200mL of DMF into a 1 L three-necked flask equipped with a reflux condenser, a thermometer, and a mechanical stirrer, stir evenly, raise the temperature to 40°C, and add acetyl chloride dropwise while stirring 13.8g (0.176mol), it takes 10min, after dripping, keep warm for 15min, TLC detects that there is no raw material point, drop 1.0g methanol, keep warm for 15min at 40°C, and recover the solvent under reduced pressure after the reaction. Add 900mL of acetone / water (2:1) mixed solution to it and stir to dissolve. After dissolution, triethylamine was added dropwise to adjust the pH to about 3. Add 300mL of acetone dropwise under slow stirring, let stand for crystallization for 1 hour after dropping, slowly cool down to 0°C, crystallize for 3 hours, filter with suction, rinse the filter cake with about 50-100mL of solvent and dry it at 70°C, that is S-acetyl-L-glutathione product 44g was obtained, the...
Embodiment 3
[0026] Add 50 g (0.16 mol) of glutathione, ZnCl 2 108mg, add 400mL trifluoroacetic acid, stir evenly, raise the temperature to 35°C, add 17.6 g (0.224mol) of acetyl chloride dropwise while stirring, and take 10 minutes. Ethanol was incubated at 35°C for 15 minutes, and the solvent was recovered under reduced pressure after the reaction. Add 900mL of acetone / water (2:1) mixed solution to it and stir to dissolve. After dissolving, trimethylamine was added to adjust the pH to about 3. Add 400mL of acetone dropwise under slow stirring, let stand for crystallization for 1 hour after dropping, slowly cool down to 0°C, crystallize for 3 hours, filter with suction, rinse the filter cake with about 50-100mL of solvent and dry it at 70°C, that is Obtain S-acetyl-L-glutathione product 48g, product yield 85.7%, HPLC detection product purity is greater than 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com