Novel process for synthesizing Pranlukast intermediate p-phenylbutoxybenzoic acid
A technology of phenbutoxybenzoic acid and methyl phenbutoxybenzoate, applied in the field of pharmaceutical synthesis, can solve the problems such as the preparation method of starting material 4-chloro-1-butanol is not described, and achieves low cost, The effect of safe reaction and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
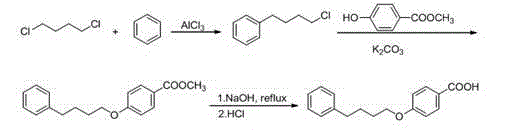
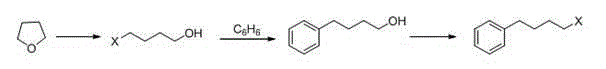
[0041] a. Synthesis of 4-phenylchlorobutane
[0042] In a 100 mL three-necked flask, add dry 1,4-dichlorobutane (15 mL) and dry benzene (5 mL), stir in an ice bath at 4~5 oC, add aluminum trichloride (2.26 g). After the reaction, slowly add 10 mL of ice water and concentrated hydrochloric acid mixture (volume ratio: 5:1), stir for 10 min, separate the organic phase, extract the water phase with benzene, combine the organic phases, and wash twice with 20 mL of water , the resulting organic phase was treated with MgSO 4 Dry, filter, and remove unreacted benzene and 1,4-dichlorobutane from the filtrate under reduced pressure, and the obtained light yellow transparent liquid is 4-phenylchlorobutane.
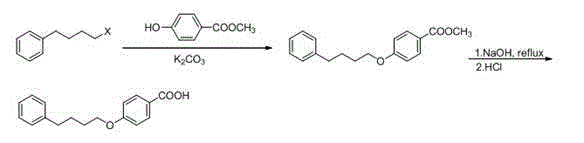
[0043] b. Synthesis of methyl p-phenylbutoxybenzoate
[0044] In a 100 mL three-necked flask, add the above product 4-phenylchlorobutane, DMF (15 mL). Under mechanical stirring, sequentially add methyl p-hydroxybenzoate (0.6 g, 3.95 mmol), potassium carbonate (1.1 g, 7.9 mmol), N...
Embodiment 2
[0048] a. Synthesis of 4-phenylchlorobutane
[0049] In a 100 mL three-neck flask, add dry 1,4-dichlorobutane (20 mL) and dry benzene (5 mL), stir in an ice bath at 4-5 oC, and add aluminum trichloride (2.9 g) in batches . After the reaction, slowly add 10 mL of ice water and concentrated hydrochloric acid mixture (volume ratio: 5:1), stir for 10 min, separate the organic phase, extract the water phase with benzene, combine the organic phases, wash twice with 20 mL of water, The obtained organic phase was treated with MgSO 4 Dry, filter, and remove unreacted benzene and 1,4-dichlorobutane from the filtrate under reduced pressure, and the obtained light yellow transparent liquid is 4-phenylchlorobutane.
[0050] b. Synthesis of methyl p-phenylbutoxybenzoate
[0051] In a 100 mL three-necked flask, add the above product 4-phenylchlorobutane, DMF (15 mL). Under mechanical stirring, sequentially add methyl p-hydroxybenzoate (0.6 g, 3.95 mmol), potassium carbonate (1.1 g, 7.9 mmo...
Embodiment 3
[0055] a. Synthesis of 4-phenylchlorobutane
[0056] In a 100 mL three-necked flask, add 25 mL of dry 1,4-dichlorobutane and 5 mL of dry benzene, stir in an ice bath at 10 °C, and add aluminum trichloride (3.76 g) in batches. After the reaction, slowly add 10 mL of ice water and concentrated hydrochloric acid mixture (volume ratio: 5:1), stir for 10 min, separate the organic phase, extract the water phase with benzene, combine the organic phases, wash twice with 20 mL of water, The obtained organic phase was treated with MgSO 4 Dry, filter, and remove unreacted benzene and 1,4-dichlorobutane from the filtrate under reduced pressure, and the obtained light yellow transparent liquid is 4-phenylchlorobutane.
[0057] b. Synthesis of methyl p-phenylbutoxybenzoate
[0058] In a 100 mL three-necked flask, add the above product 4-phenylchlorobutane, DMF (15 mL). Under mechanical stirring, sequentially add methyl p-hydroxybenzoate (0.6 g, 3.95 mmol), potassium carbonate (1.1 g, 7.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com