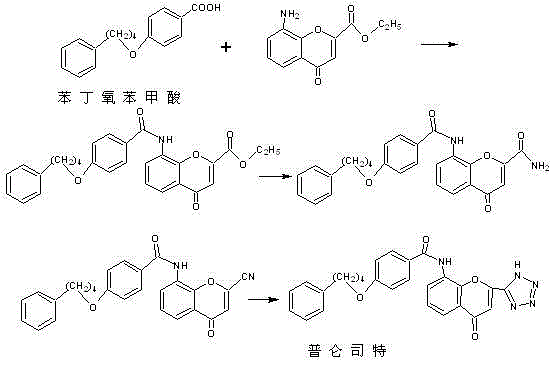
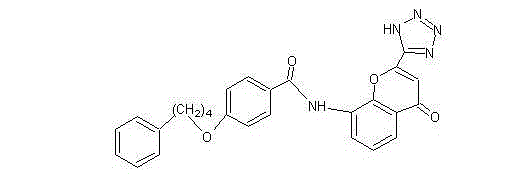
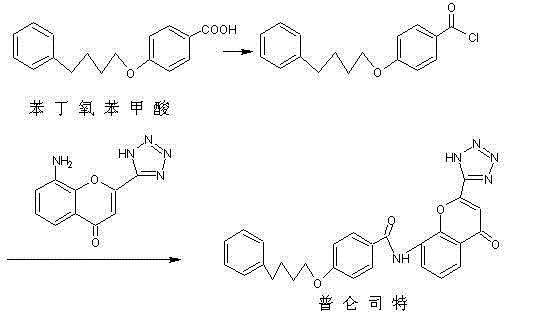
A kind of refining purification method of pranlukast intermediate phenbutoxybenzoic acid
A technology of phenbutoxybenzoic acid and phenbutoxybenzoic acid, which is applied in the refining of pharmaceutical intermediates and the refining of chemical drug prankast intermediates, can solve the problem that the purity of prankast cannot meet the quality standard requirements, etc. problem, to achieve the effect of low price, high quality and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In the three-necked flask, add 50g of crude phenylbutoxybenzoic acid (the impurity content of dicarboxylic acid is 0.8%) and 500ml of ethanol in turn, heat up to 50°C under stirring to dissolve, add 30.4g of potassium hydroxide aqueous solution (10.4g of potassium hydroxide Dissolved in 20ml of water), slowly cooled to below 20°C, and crystals were precipitated. After suction filtration, about 5 g of the filter cake was dried, and the HPLC spectrum showed that the dicarboxylic acid impurity content was 8.5%.
[0034] The mother liquor is distilled to recover ethanol, and 500ml of water is added to the concentrate to dissolve it. Cool to 5~10°C, adjust PH=1.45 with concentrated hydrochloric acid, and precipitate crystals. Suction filtration, drying the filter cake to obtain 44.2 g of the product. The HPLC content is 99.7%, and the dicarboxylic acid impurity amount is 0.09%. The refined yield is 88.4%.
Embodiment 2
[0036] In the three-necked flask, add 50g of crude phenylbutoxybenzoic acid (the impurity content of dicarboxylic acid is 0.28%) and 1000ml of methanol in sequence, heat up to 30°C under stirring to dissolve, add 19g of 25% ammonia water, cool to below 20°C to precipitate crystals . Suction filtration, about 6g of the filter cake after drying, HPLC spectrum shows wherein dicarboxylic acid impurity content is 2.4%.
[0037] Distill the mother liquor to recover methanol, add 500ml of water to the concentrate to dissolve it, adjust the pH to 3.82 with acetic acid, and precipitate crystals. After suction filtration, the filter cake was dried to obtain 43.1 g of the product, the HPLC content was 99.6%, the dicarboxylic acid impurity content was 0.05%, and the refined yield was 86.2%.
Embodiment 3
[0039] In the three-necked flask, add 50g of crude phenylbutoxybenzoic acid (the impurity content of dicarboxylic acid is 0.28%) and 500ml of n-butanol in sequence, heat up to 70°C under stirring to dissolve, add 31.5g of 20% methanolic ammonia solution, and cool to precipitate crystals. After suction filtration, the filter cake was about 4.2g after drying, and the HPLC spectrum showed that the dicarboxylic acid impurity content was 4.5%.
[0040] Distill the mother liquor to recover the solvent, add 500ml of water to the concentrate to dissolve it, adjust the pH to 1.74 with concentrated hydrochloric acid, and precipitate crystals. After suction filtration, the filter cake was dried to obtain 45.6 g of the product, the HPLC content was 99.6%, the dicarboxylic acid impurity content was 0.04%, and the refined yield was 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com