Preparation method of pranlukast intermediate
An intermediate, the technology of hydroxyacetophenone, applied in the field of preparation of pranlukast intermediate, can solve problems such as environmental pollution, achieve the effect of increasing reaction yield, reducing reaction activity, and streamlining process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of pranlukast intermediate, comprising the following steps:
[0042] S1. Weigh 100g of 4-phenylbutoxybenzoic acid and put it into a three-necked flask with a capacity of 1L, add 3-amino-2-hydroxyacetophenone, 4-phenylbutoxybenzoic acid and 3-amino-2 -The mass ratio of hydroxyacetophenone is 1:0.56, then add 200g Polyethylene Glycol-400 to dissolve;
[0043] S2. Then add the phosphotungstic acid catalyst, the mass ratio of the added phosphotungstic acid catalyst to 4-phenylbutoxybenzoic acid is 0.008:1, stir and mix well, heat to 150°C under the condition of nitrogen protection, keep warm for 10h ;
[0044] S3. After the reaction is completed, cool to 8°C, add 400mL ether for extraction, let stand for stratification, keep the lower polyethylene glycol layer, spin-evaporate the separated upper ether layer, and recover the phosphotungstic acid catalyst;
[0045] S4, adding dilute nitric acid with a concentration of 0.05wt% to the polyethylene glycol ...
Embodiment 2-10
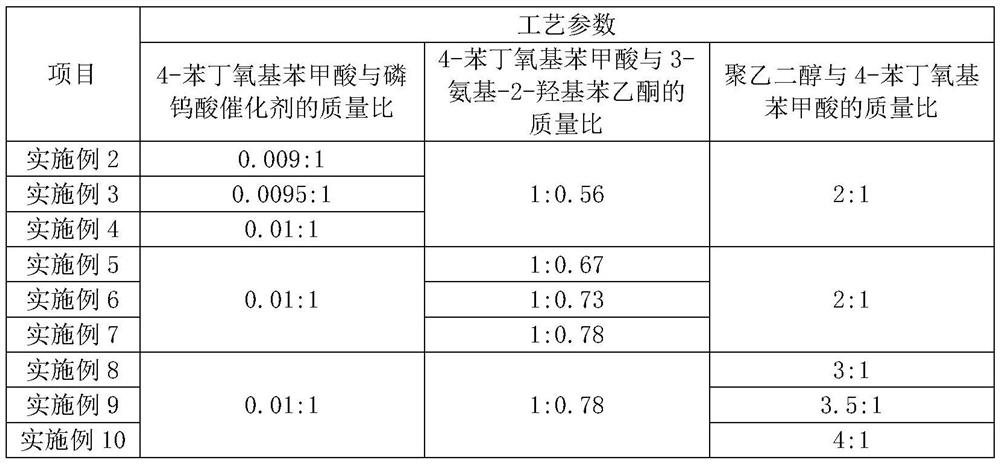
[0048] Embodiments 2-10 all relate to a preparation method of a pranlukast intermediate, based on Example 1, the difference is: the mass ratio of 4-phenylbutoxybenzoic acid and phosphotungstic acid catalyst in step S1, The mass ratio of 4-phenylbutoxybenzoic acid and 3-amino-2-hydroxyacetophenone is different from that of polyethylene glycol and 4-phenylbutoxybenzoic acid, and the specific values are shown in the following table:
[0049] Table 1. The mass ratio of 4-phenylbutoxybenzoic acid to phosphotungstic acid catalyst, the mass ratio of 4-phenylbutoxybenzoic acid to 3-amino-2-hydroxyacetophenone and polyethylene glycol to 4- The concrete numerical value of the mass ratio of phenylbutoxybenzoic acid:
[0050]
Embodiment 11-18
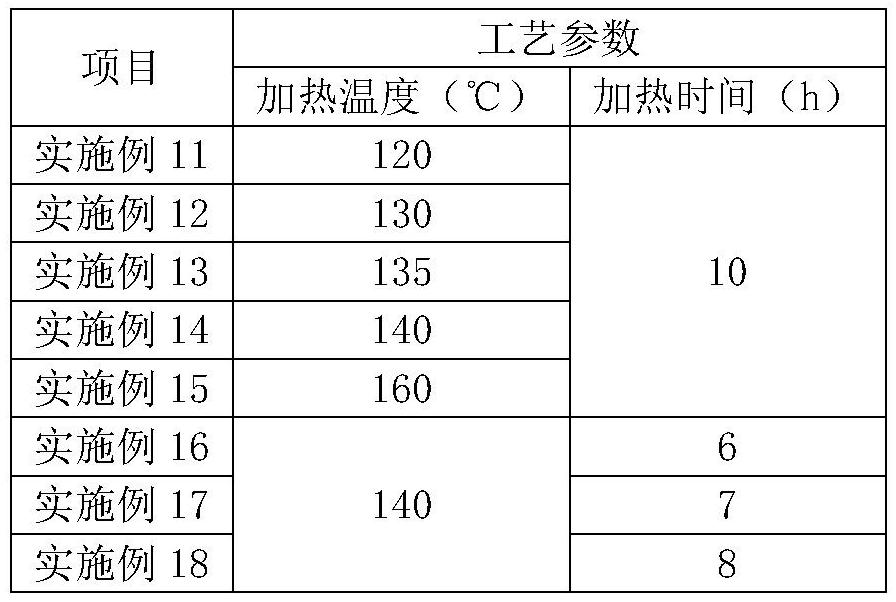
[0052]Examples 11-18 all relate to a preparation method of a pranlukast intermediate, based on Example 10, the difference is that the reaction temperature and reaction time in step S2 are different, and the specific values are shown in the following table:
[0053] Reaction temperature and reaction time in table 2. step S2:
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com