New preparation method of Pranlukast
A reaction formula, the technology of phenylbutoxyl, which is applied in the field of medicine, can solve the problems of unreported starting material synthesis method, unfavorable operation and safe production, and many reaction steps in the synthesis process, and achieve novel routes, simple equipment, and advanced technology. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
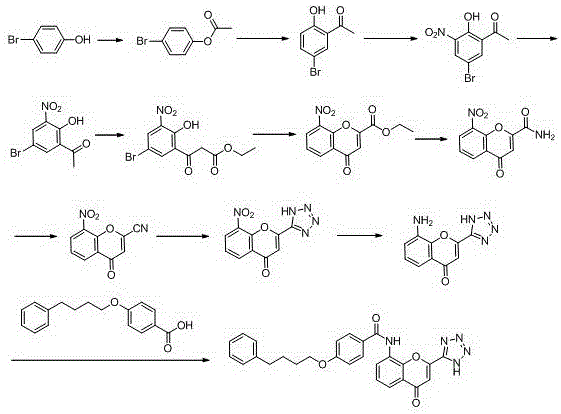
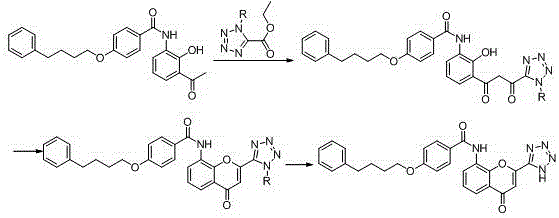
Image
Examples
Embodiment 1
[0058] Embodiment 1: the preparation (I) of 3-acetamido-4-acetoxybenzenesulfonic acid
[0059] Take 2-aminophenol-4-sulfonic acid (189g), acetic anhydride (224g), methanol 400g, concentrated sulfuric acid 10ml, reflux reaction for 4h. After the reaction, recover methanol under reduced pressure, cool to room temperature, add The saturated sodium bicarbonate solution was adjusted to pH 9-10, extracted with 400 ml of ethyl acetate, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered under reduced pressure to obtain 264 g of light yellow solid with a yield of 97%.
Embodiment 2
[0060] Example 2: Synthesis of 3-amino-2-hydroxyacetophenone.
[0061] Take 136 g of 3-acetamido-4-acetoxybenzenesulfonic acid, dissolve it in 300 ml of nitrobenzene, add 133 g of anhydrous aluminum trichloride, heat and reflux for 5 h, and recover most of the solvent under reduced pressure after the reaction is completed. Cool to room temperature, under ice bath, slowly add the reaction solution into 30% dilute hydrochloric acid, stir at room temperature for 2 hours, slowly raise the temperature to reflux, react for 4 hours, after the reaction, cool the reaction solution to room temperature, add 20% hydrogen hydroxide to the reaction system Sodium solution adjusts pH 9-10. Add 300 ml of chloroform for extraction, wash with water until neutral, dry over anhydrous sodium sulfate, filter, and recover the filtrate under reduced pressure. The residue is recrystallized with petroleum ether: ethyl acetate = 1:3 to obtain 65.7 g of a light yellow solid, with a yield of 87%.
Embodiment 3
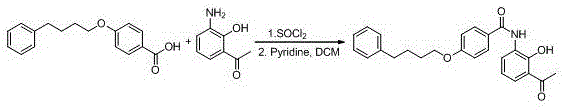
[0062] Example 3: Preparation of 2-acetyl-6-[4-(4-phenylbutoxy)benzamido]phenol (III)
[0063] Take 270 g of 4-(phenylbutoxy)benzoic acid, add 270 g of thionyl chloride, and keep warm at 50°C for 3 hours. After the reaction, unreacted thionyl chloride is recovered under reduced pressure. The thionyl chloride was purged with nitrogen gas. Cool to room temperature and add 270g of dichloromethane for later use. Weigh 151g of 3-amino-2-hydroxyacetophenone and dissolve it in 200g of dichloromethane, add 160g of pyridine, add dropwise the dichloromethane solution of 4-(phenylbutoxy)benzoyl chloride under ice-cooling, the dropwise addition process The medium temperature does not exceed 10°C. After the dropwise addition is completed, keep the temperature at 10°C for 2 hours. After the reaction, add dilute hydrochloric acid to the reaction system, adjust the pH to 2-3, separate the liquids, and wash the dichloromethane layer with water until it is neutral and anhydrous. It was dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com