Pranlukast solid dispersion and preparation method thereof
A solid dispersion and carrier technology, applied in the directions of active ingredients of heterocyclic compounds, drug combinations, respiratory diseases, etc., can solve the problems of low bioavailability, poor viscosity and water solubility, achieve good reproducibility, reduce pollution, reduce The effect of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Pranlukast 10% lactose 50% microcrystalline cellulose 39% Colloidal silica 0.5% Magnesium stearate 0.5%
[0021] Preparation Process:
[0022] The vilazodone hydrochloride, lactose, and microcrystalline cellulose passed through an 80-mesh sieve are uniformly mixed, and then colloidal silicon dioxide and magnesium stearate are added and mixed to form tablets with a weight of about 200 mg.
[0023]
Embodiment 2
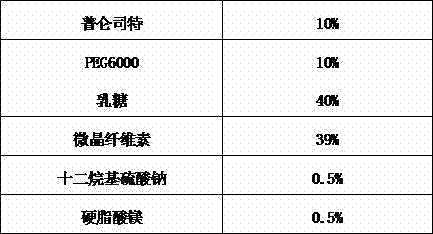
[0025]
[0026] Preparation Process:
[0027] Put PEG6000 in a 70°C water bath and heat to melt. Dissolve pranlukast in dichloromethane-methanol solution containing surfactant, dissolve it completely by heating, add this solution into the melted solid medium, and mix well. The solvent of the above mixture is dried by spray drying or vacuum drying. After cooling, place it in a mortar and grind it properly to obtain the solid dispersion of pranlukast. The obtained solid dispersion was added with lactose, MCC, and magnesium stearate, mixed uniformly, and then pressed into tablets, with a tablet weight of about 200 mg.
[0028]
Embodiment 3
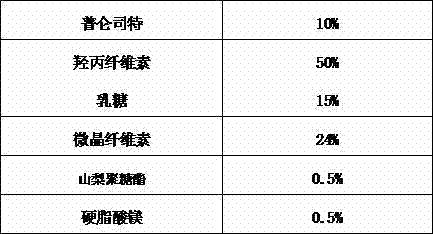
[0030]
[0031] Preparation Process:
[0032] Put hydroxypropyl cellulose in a 70°C water bath and heat to melt. Dissolve pranlukast with dichloromethane-methanol solution containing sorbitan ester, dissolve it completely by heating, add this solution into the melted solid medium, and mix well. The solvent of the above mixture is dried by spray drying or vacuum drying. After cooling, place it in a mortar and grind it properly to obtain the solid dispersion of pranlukast. The obtained solid dispersion was added with lactose, MCC, and magnesium stearate, mixed uniformly, and then pressed into tablets, with a tablet weight of about 200 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com