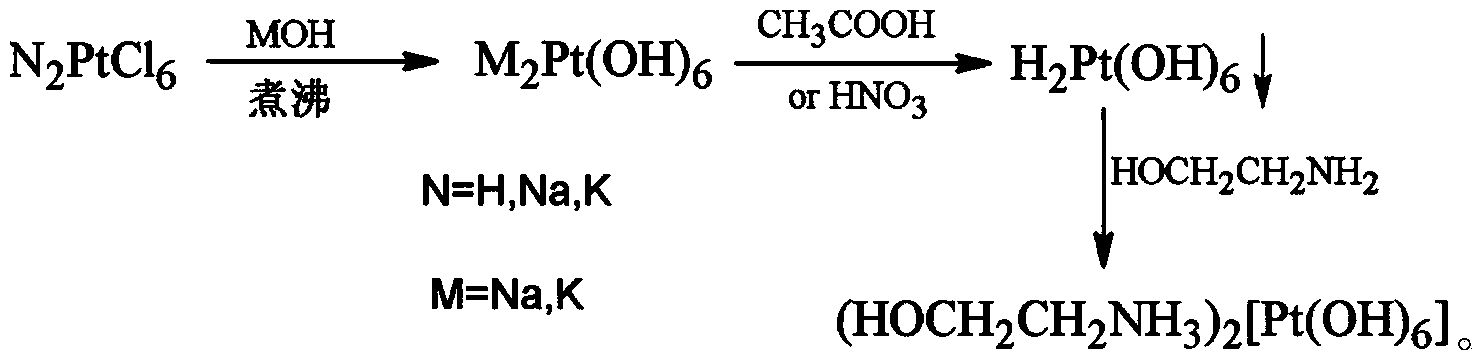Method for preparing stable 6-hydroxyl platinum (IV) acid diethanolamine water solution
A technology of aqueous solution and ethanolamine, which is applied in the field of chemical catalysis, can solve problems such as difficulty in loading, reduction of dispersion and adhesion of active ingredients, and inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0011] Example 1a: Intermediate H 2 Pt(OH) 6 preparation of
[0012] Take commercially available K 2 PtCl 6 48.6g (0.1mol) in 1000mL water, stir well, add 500mL aqueous solution containing 67.2g (1.2mol) of potassium hydroxide, heat to boil until the color of the solution turns light yellow-green, cool to room temperature, adjust the pH of the solution with acetic acid =5.0, a large amount of pale yellow precipitate H was precipitated 2 Pt(OH) 6 , collected by filtration, washed with water until no Cl was detected - Dry at 60°C to obtain 29.1 g of light yellow solid powder with a yield of 97.0%.
[0013] Structural determination data: Elemental analysis: Pt64.9%, H2.75% is consistent with the theoretical value (Pt65.2%H2.70%), in line with H 2 Pt(OH) 6 Structure.
[0014] b:(HOCH 2 CH 2 NH 3 ) 2 [Pt(OH) 6 ] for the preparation of an aqueous solution
[0015] Take 15.0 g (50.17 mmol) H 2 Pt(OH) 6 , add 100mL of 6.20% ethanolamine aqueous solution (equivalent to...
Embodiment 2
[0019] a: Intermediate H 2 Pt(OH) 6 preparation of
[0020] Take commercially available Na 2 PtCl 6 Dissolve 90.8g (0.2mol) in 1200mL water, add 800mL aqueous solution containing 96g (2.4mol) sodium hydroxide, heat to boil until the color of the solution turns light yellow-green, cool to room temperature, adjust the pH of the reaction solution to 4.0 with nitric acid, and precipitate A large amount of pale yellow precipitate H 2 Pt(OH) 6 , collected by filtration, washed with water until no Cl was detected - Dry at 60° C. to obtain 58.7 g of light yellow solid powder with a yield of 98.0%.
[0021] Structure determination data: Elemental analysis: Pt64.7%, H2.79% are consistent with theoretical values (Pt65.2%H2.69%). Complies with H 2 Pt(OH) 6 Structure.
[0022] b: product (HOCH 2 CH 2 NH 3 ) 2 [Pt(OH) 6 ] preparation
[0023]Take 45 g (150.5 mmol) H 2 Pt(OH) 6 , add 100mL of 18.5% ethanolamine aqueous solution (equivalent to 303mmol), heat to dissolve, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com