New preparation method of 3-amino-2-hydroxyphenylacetone
A technology of hydroxyacetophenone and a new method, applied in the field of medicine, can solve the problems of low yield, long synthetic route and the like, and achieve the effects of high purity, simple equipment and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
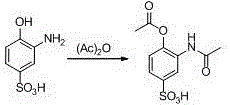
[0022] Embodiment 1: the preparation (I) of 3-acetamido-4-acetoxybenzenesulfonic acid
[0023] Take 2-aminophenol-4-sulfonic acid (189g), acetic anhydride (224g), methanol 400g, concentrated sulfuric acid 10ml, reflux reaction for 4h. After the reaction, recover methanol under reduced pressure, cool to room temperature, add The saturated sodium bicarbonate solution was adjusted to pH 9-10, extracted with 400 ml of ethyl acetate, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered under reduced pressure to obtain 264 g of light yellow solid with a yield of 97%.
Embodiment 2
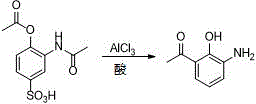
[0024] Example 2: Synthesis of 3-amino-2-hydroxyacetophenone (II)
[0025] Take 136g of 3-acetamido-4-acetoxybenzenesulfonic acid, dissolve it in 300ml of chloroform, add 133g of anhydrous aluminum trichloride, and heat to reflux for 5h. After the reaction is over, recover most of the solvent under reduced pressure and cool to At room temperature, under an ice bath, the reaction solution was slowly added to 30% dilute hydrochloric acid, stirred at room temperature for 2 hours, slowly raised to reflux, and reacted for 4 hours. After the reaction, the reaction solution was cooled to room temperature, and 20% sodium hydroxide solution was added to the reaction system Adjust pH9-10. Add 300 ml of chloroform for extraction, wash with water until neutral, dry over anhydrous sodium sulfate, filter, and recover the filtrate under reduced pressure. The residue is recrystallized with petroleum ether: ethyl acetate = 1:3 to obtain 65.7 g of a light yellow solid, with a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com