Method for synthesizing drug pranlukast from tetrahydrofuran path
A technology for synthesizing drugs and tetrahydrofuran, applied in directions such as organic chemistry, can solve problems such as difficult industrialization, difficulty in tetrazolium, and no mention of synthetic methods, and achieve the effects of easy availability of raw materials, safe reaction, and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
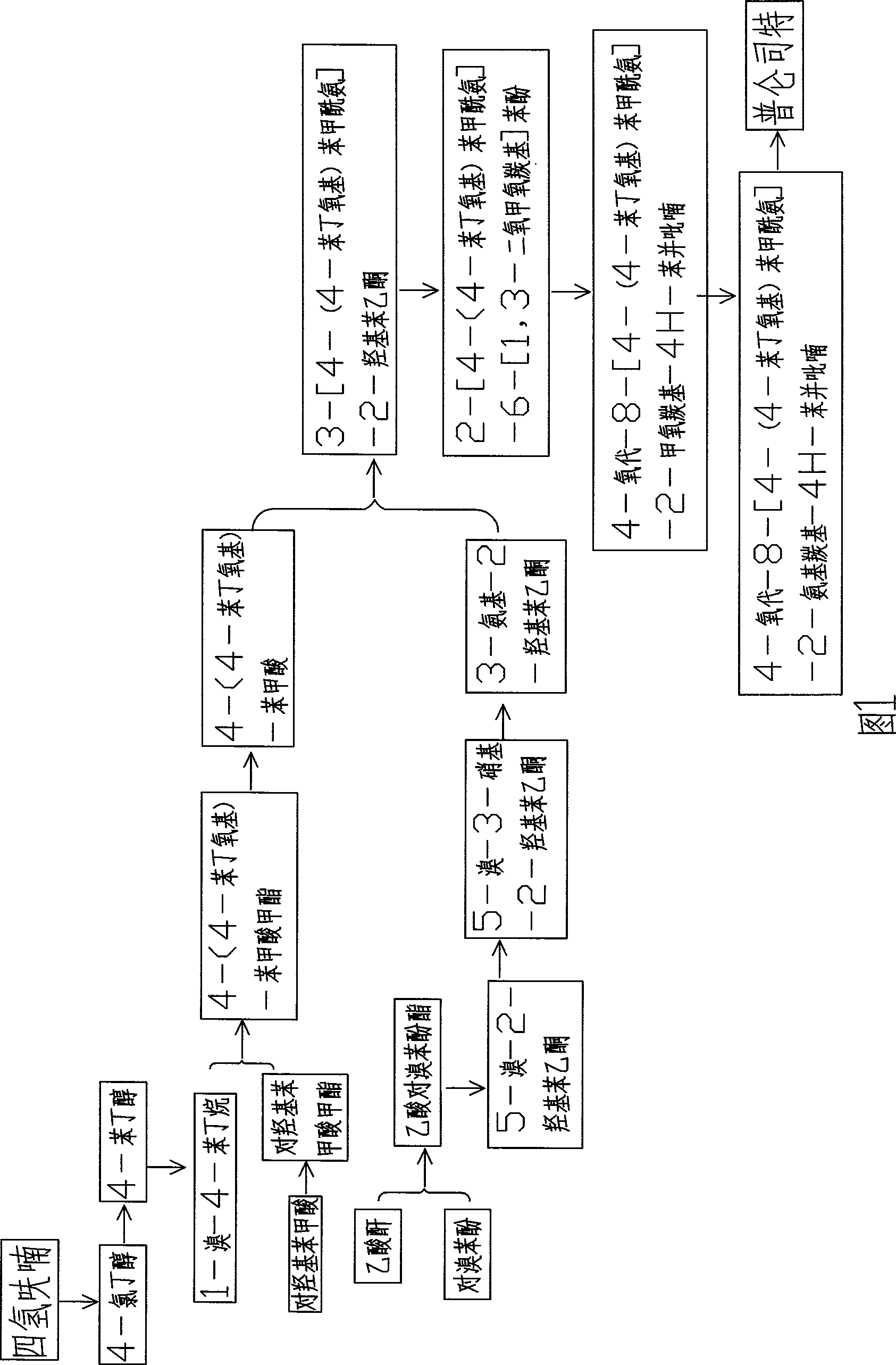
[0081] A method for synthesizing drug pranlukast from tetrahydrofuran approach, the synthetic route of the method is as follows:
[0082]
[0083] With reference to the process flow diagram shown in Figure 1, the above-mentioned synthetic method may further comprise the steps:
[0084] a. Synthesis of 4-chlorobutanol A
[0085] Add 144 g of THF and 200 g of concentrated hydrochloric acid into a three-neck flask, react at 60°C for 12 h, cool, extract with dichloromethane, remove the solvent, and distill under reduced pressure to obtain product A, namely 4-chlorobutanol.
[0086] b. Synthesis of 4-phenylbutanol B
[0087] Add 100ml of benzene and 44.3g of aluminum trichloride to the four-neck flask, add A30g dropwise at 12°C, and react for 7.5h. Pour into ice water, separate liquid, remove solvent, and distill under reduced pressure to obtain colorless transparent liquid B.
[0088] c. Synthesis of 1-bromo-4-phenylbutane C
[0089] Add 0.38g of B and 1.08g of 40% hydrobro...
Embodiment 2— Embodiment 5
[0117] The difference of embodiment 2-5 and embodiment 1 is only that the consumption of substance is different, the condition of reaction is different, as shown in the table; In addition, the amount of each step output substance is also different (not shown in the table) and each step Not all of the output was used in subsequent steps.
[0118] Other contents of the preparation methods of the above-mentioned embodiments are the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com