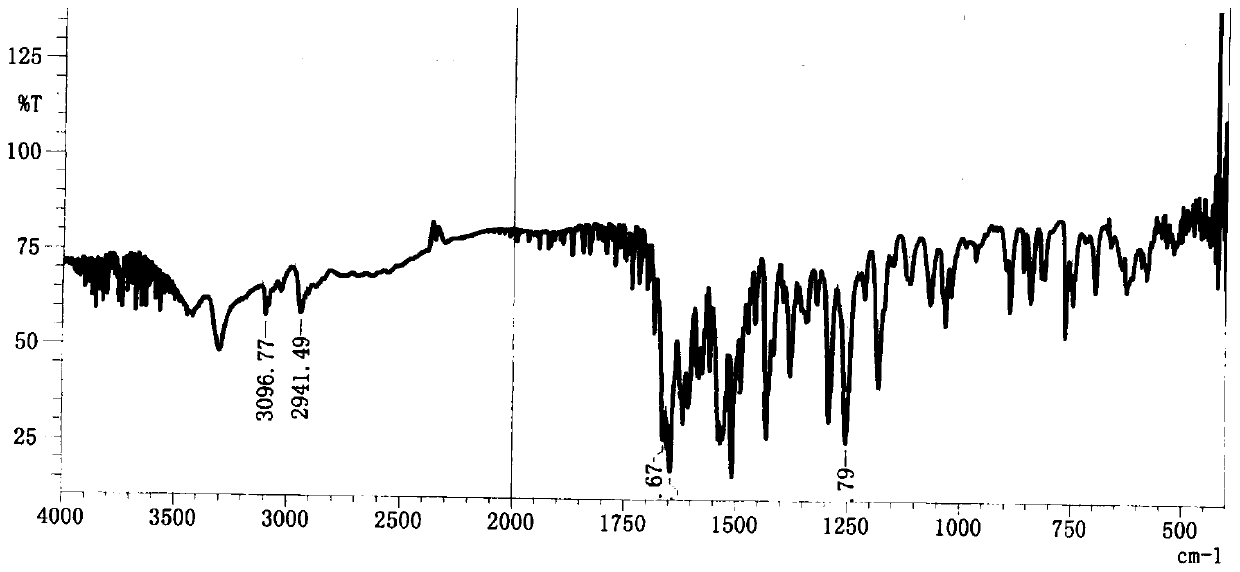
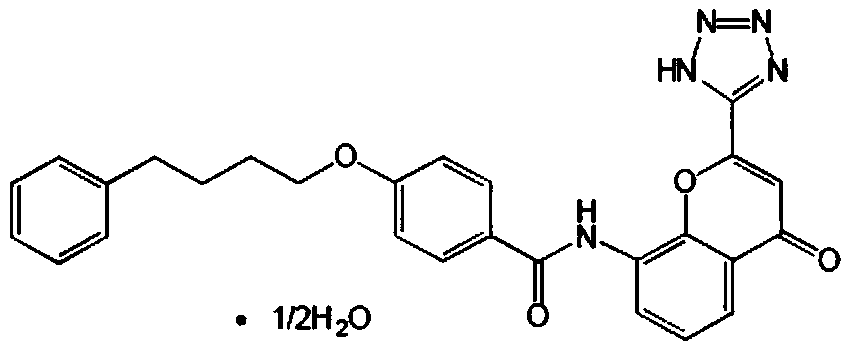
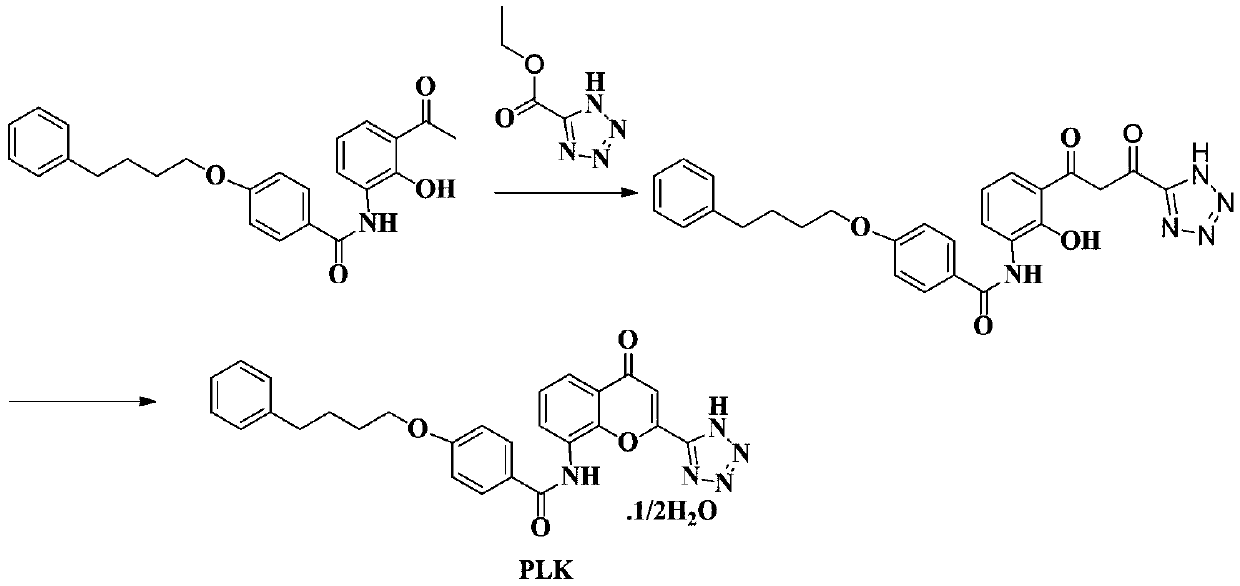
A kind of preparation method of pranlukast
A reaction and solvent technology, applied in the field of medicine, can solve problems such as long synthetic routes, many synthetic steps, and long routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of ethyl 2-iodo-3-(4-bromobenzamide)benzoate
[0035] Add 20.1g (0.1mol) of 4-bromobenzoic acid and 100mL of ethyl acetate to a 250mL three-necked flask, start stirring and heat up to 50-60°C, add 13.1g (0.11mol) of thionyl chloride dropwise, after the dropwise addition is completed , continue to stir for 0.5 hours, then concentrate to dryness, add 100mL ethyl acetate to the residue and shake well for later use, add 29.1g (0.1mol) ethyl 3-amino-2-iodobenzoate and 300mL acetic acid to a 500mL three-necked flask Ethyl ester and 11.85g (0.15mol) pyridine, add the above standby solution dropwise at 20-30°C (dropping temperature is generally in the range value), after the drop is completed, the temperature is raised to 80°C, and the reaction is kept for 5 hours. TLC detects that the reaction is complete. Stir and cool down to 20°C, precipitate the product, and filter to obtain 45.03g of off-white solid with a yield of 95.06% and a purity of 99.2%.
[0036] (2)...
Embodiment 2
[0041] (1) Synthesis of ethyl 2-iodo-3-(4-bromobenzamide)benzoate
[0042] Add 20.1g (0.1mol) of 4-bromobenzoic acid and 100mL of ethyl acetate to a 250mL three-necked flask, start stirring and raise the temperature to 60°C, add 13.1g (0.11mol) of thionyl chloride dropwise, after the dropwise addition is complete, continue Stir for 0.5 hours, then concentrate to dryness, add 100mL ethyl acetate to the residue and shake well for later use, add 29.1g (0.1mol) ethyl 3-amino-2-iodobenzoate and 300mL ethyl acetate to a 500mL three-necked flask and 15.15g (0.15mol) of triethylamine, add the above-mentioned standby solution dropwise between 20 and 30°C, raise the temperature to 80°C after dropping, keep the temperature for 5h, TLC detects that the reaction is complete, stir and cool down to 20°C, precipitate the product, filter , to obtain 40.1 g of off-white solid with a yield of 84.65% and a purity of 98.3%.
[0043] (2) Synthesis of 4-bromo-N-(4-oxo-2-(1H-tetrazol-5-yl)-4H-benzop...
Embodiment 3
[0048] (1) Synthesis of ethyl 2-iodo-3-(4-bromobenzamide)benzoate
[0049] Add 20.1g (0.1mol) of 4-bromobenzoic acid and 100mL of tetrahydrofuran to a 250mL three-necked flask, start stirring and heat up to 60°C, add 13.1g (0.11mol) of thionyl chloride dropwise, and continue stirring for 0.5 hour, and then concentrated to dryness, 100mL tetrahydrofuran was added to the residue and shaken for subsequent use, and 29.1g (0.1mol) ethyl 3-amino-2-iodobenzoate and 300mL tetrahydrofuran and 11.85g (0.15mol ) pyridine, add the above standby solution dropwise at 20-30°C, heat up to 70°C after dropping, keep warm for 4-5 hours, TLC detects that the reaction is complete, stir and cool down to 20°C, precipitate the product, filter to obtain 44.32g off-white Solid, yield 93.56%, purity 99.47%.
[0050] (2) Synthesis of 4-bromo-N-(4-oxo-2-(1H-tetrazol-5-yl)-4H-benzopyrone-8-yl)benzamide
[0051] In a 500mL three-necked flask, add 10.48g (0.093mol) 1-(1H-tetrazol-5-yl)ethanone, 45.03g (0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com