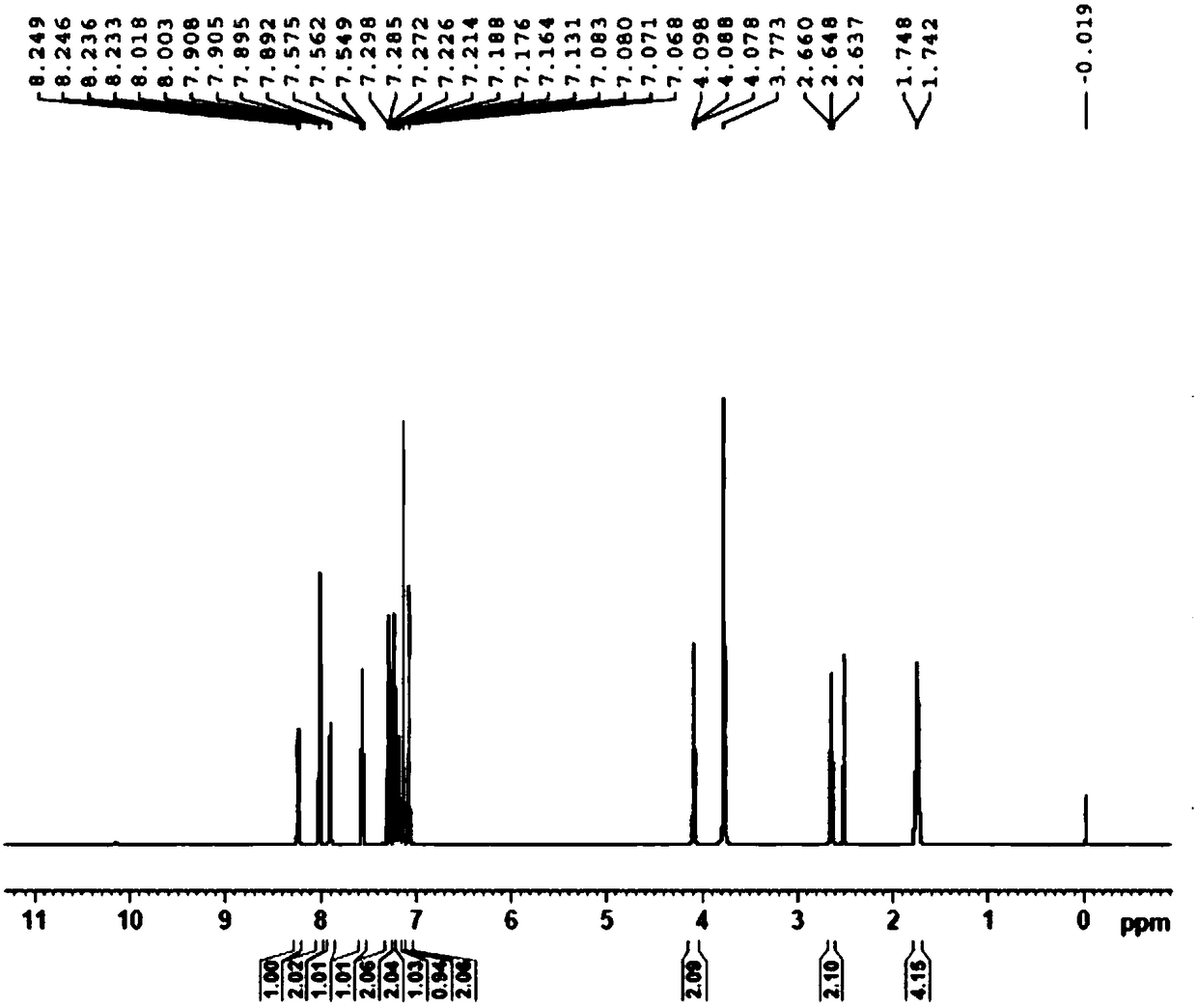
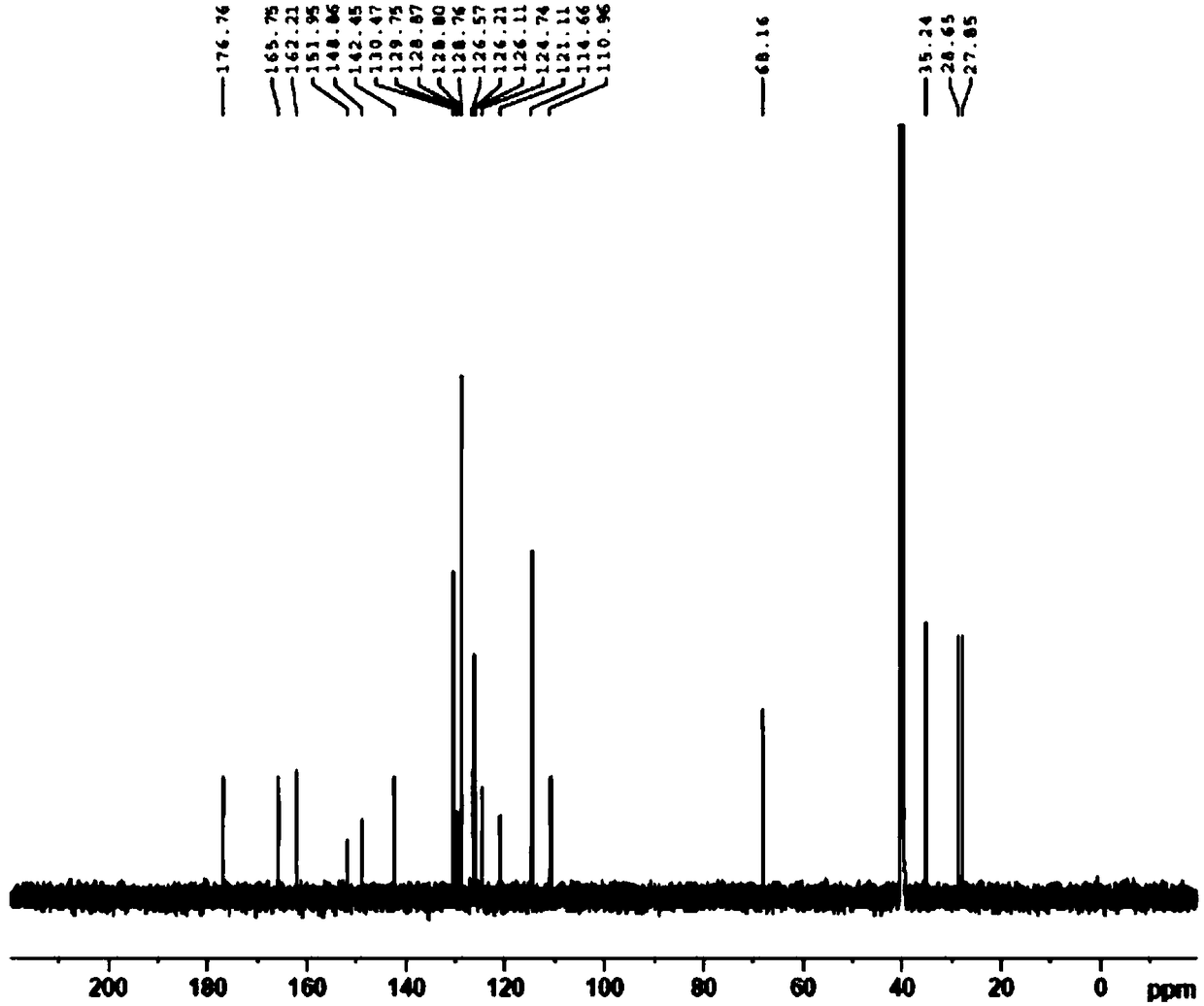
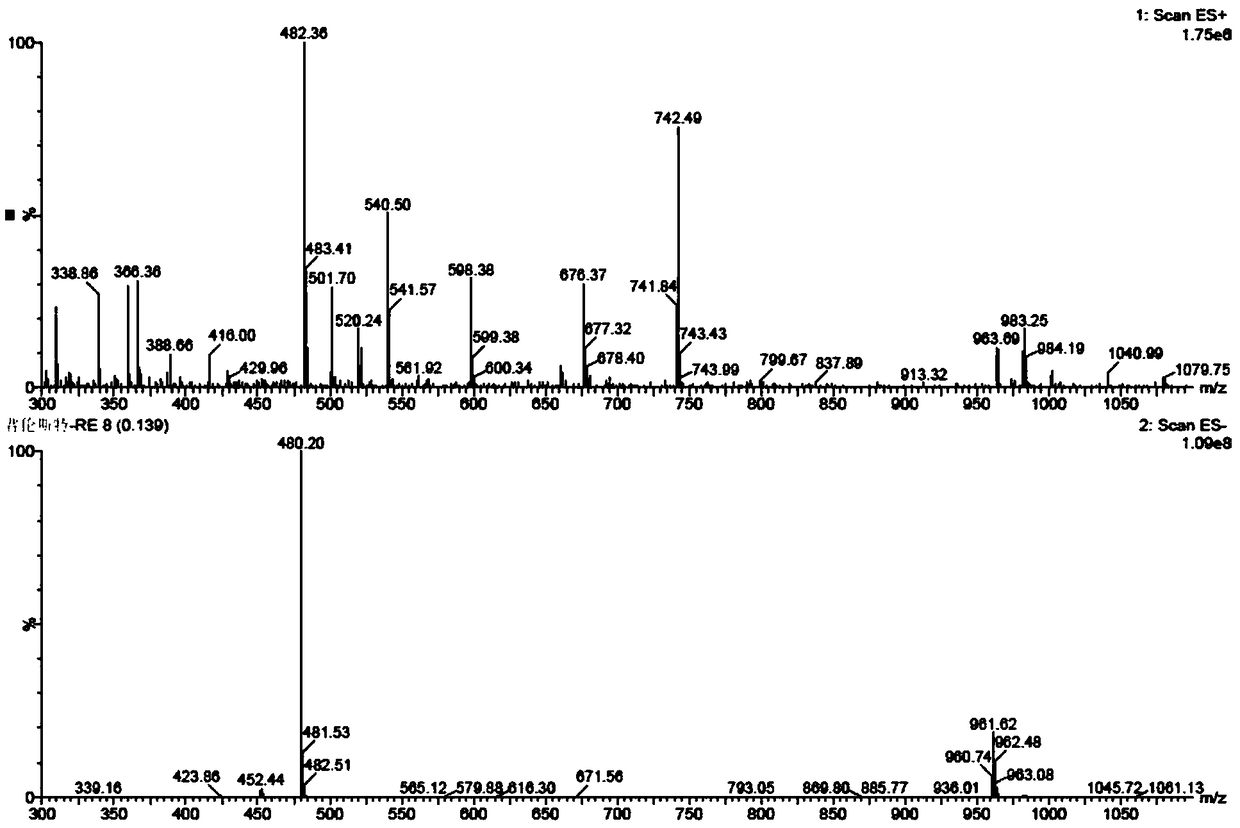
Preparation method for pranlukast
A technology of condensation reaction and phenylbutoxy group, which is applied in the field of preparation of Pronst, can solve the problems of high energy consumption and complicated operation steps, and achieve the effect of low energy consumption and reduction of preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention provides a kind of preparation method of Prenzast, comprising the following steps:
[0028] Under the protection of nitrogen and the action of an organic base, 3-[4-(4-phenylbutoxy)benzamido]-2-hydroxyacetophenone and ethyl tetrazolecarboxylate were subjected to Claisen condensation reaction in an organic solvent , to obtain a condensation reaction solution; the reaction solution includes a compound having a structure shown in formula I; the organic solvent includes one of N, N-dimethylformamide, dimethylacetamide and N-methylpyrrolidone species or several;
[0029]
[0030] Mixing the condensation reaction solution and the acid solution, the compound having the structure shown in formula I undergoes a cyclization reaction to obtain Prenzast.
[0031] In the present invention, under the protection of nitrogen and the action of an organic base, 3-[4-(4-phenylbutoxy)benzamido]-2-hydroxyacetophenone (structure shown in formula II) and tetrazole fo...
Embodiment 1
[0045]Under nitrogen atmosphere, potassium tert-butoxide (31.36g) was dissolved in DMF (160ml) under stirring, and at room temperature, 3-[4-(4-phenylbutoxy)benzamido]- 2-Hydroxyacetophenone (16.12g) was added to the obtained solution, followed by ethyl tetrazolecarboxylate (7.39g), the reaction temperature rose to 60°C, and the reaction was kept for 5 hours to obtain a condensation reaction solution.
[0046] Then cool the reaction solution to below 10°C, add dilute hydrochloric acid solution (50g concentrated hydrochloric acid + 250g water) in another reaction vessel in advance, add the condensation reaction solution to the dilute hydrochloric acid solution, and perform ring closure reaction at 30°C After 4 hours, after the reaction was completed, the product was filtered, washed with water and dried to obtain Pronstadt (4-oxo-8-[4-(4-phenylbutoxy)benzamido]-2-tetra Azol-5-yl-4H-1-benzopyran hemihydrate), the product quality is 18.5g, the yield is 96%, and the HPLC purity is...
Embodiment 2
[0049] Under a nitrogen atmosphere, potassium methoxide (19.64g) was dissolved in DMF (160ml) with stirring, and 3-[4-(4-phenylbutoxy)benzamido]-2-hydroxyl Acetophenone (16.12 g) was added to the resulting liquid, followed by ethyl tetrazolecarboxylate (7.39 g). The reaction temperature was raised to 60°C, and the reaction was kept for 5 hours to obtain a condensation reaction solution.
[0050] Then the condensation reaction solution was cooled to below 10° C., and a dilute hydrochloric acid solution (50 g of concentrated hydrochloric acid + 250 g of water) was added in advance to another reaction vessel. The condensation reaction solution was added to the dilute hydrochloric acid solution, the temperature was controlled at 30°C, and the cyclization reaction was carried out for 5 hours. After the reaction was completed, the product was filtered, washed with water and dried to obtain Prenzast. The product quality was 16.5 g, and the yield was was 85.8%, and the HPLC purity wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com