Pharmaceutical composition of pranlukast solid-dispersion with improved initial dissolution rate and the method of preparing the same
A technology of solid dispersion and composition, applied in the directions of drug combination, active ingredients of heterocyclic compounds, allergic diseases, etc., can solve the problems of sticking to gelatin, high drug elimination rate, and achieve the effect of Cmax improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0028] Embodiment 1-3: Prepare the pharmaceutical composition of pranlukast solid dispersion by using anticoagulant
[0029] A solid dispersion of prankast was prepared by thermally melting solid prankast with crospovidone, which is a water-soluble polymer, and the solid dispersion of prankast thus prepared was verified by using XRD (X-ray Diffraction) The body is amorphous.
[0030] 6 g of the solid dispersion of pranlukast specified in Table 1 and 0.06 g of anticoagulant were mixed in a double-jacketed beaker at 60° C. for 30 minutes to prepare granules. The granules were cooled at room temperature and sieved through a 20-mesh sieve, thereby obtaining a granule pharmaceutical composition of pranlukast solid dispersion.
[0031] The pharmaceutical composition of pranlukast solid dispersion is thoroughly mixed with a lubricant and filled into capsules by using a manual capsule filling machine.
[0032] Table 1
[0033]
Embodiment 4-15
[0042] Embodiment 4-15: By using different amounts of anticoagulants to prepare the pharmaceutical composition of pranlukast solid dispersion
[0043] By mixing 1 g of the same pranlukast solid dispersion as in Examples 1-3 with an anticoagulant at 60° C. in the proportions specified in Table 3, followed by the same procedure as described in Examples 1-3 To prepare the granular pharmaceutical composition of pranlukast solid dispersion.
[0044] table 3
[0045]
experiment example 1
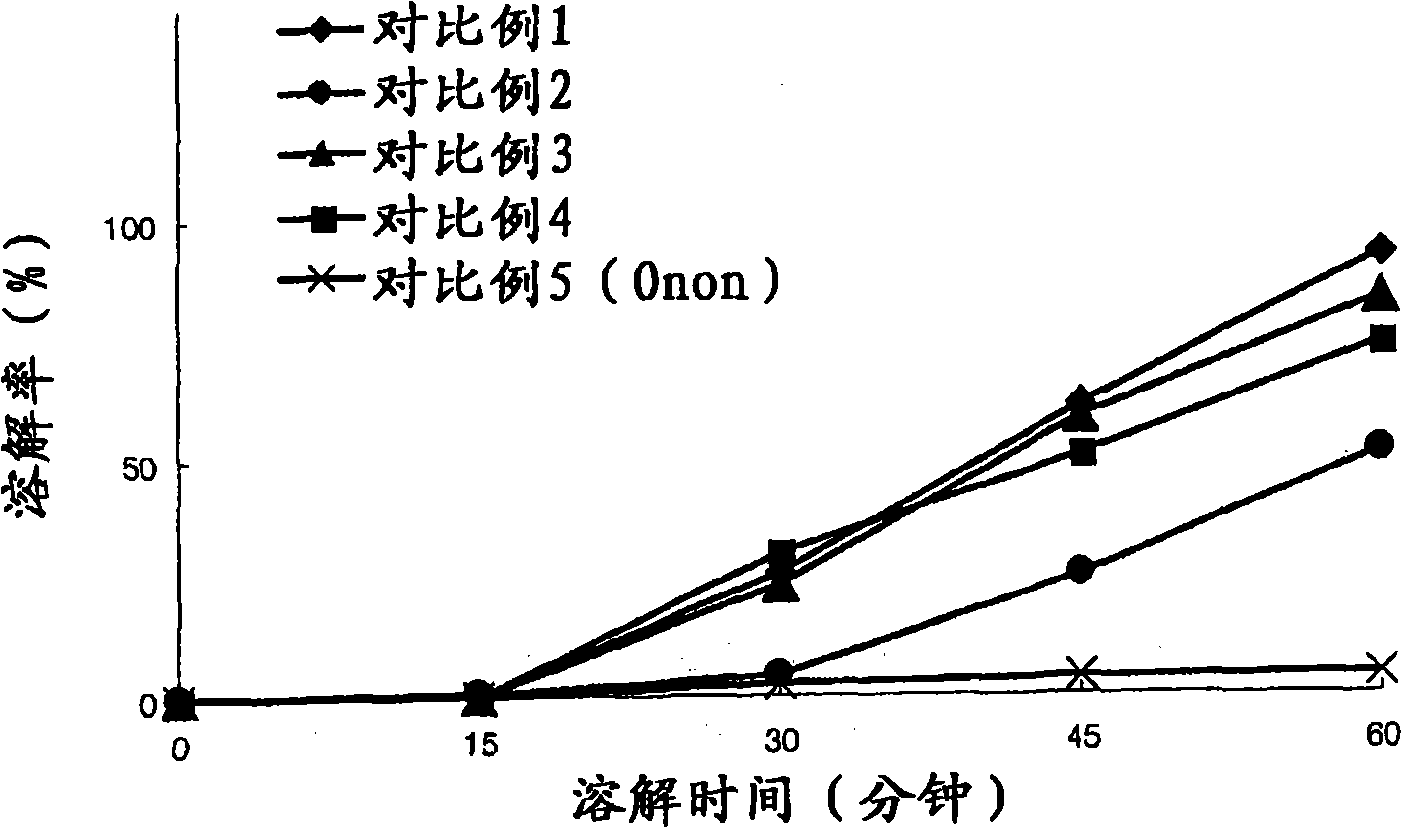
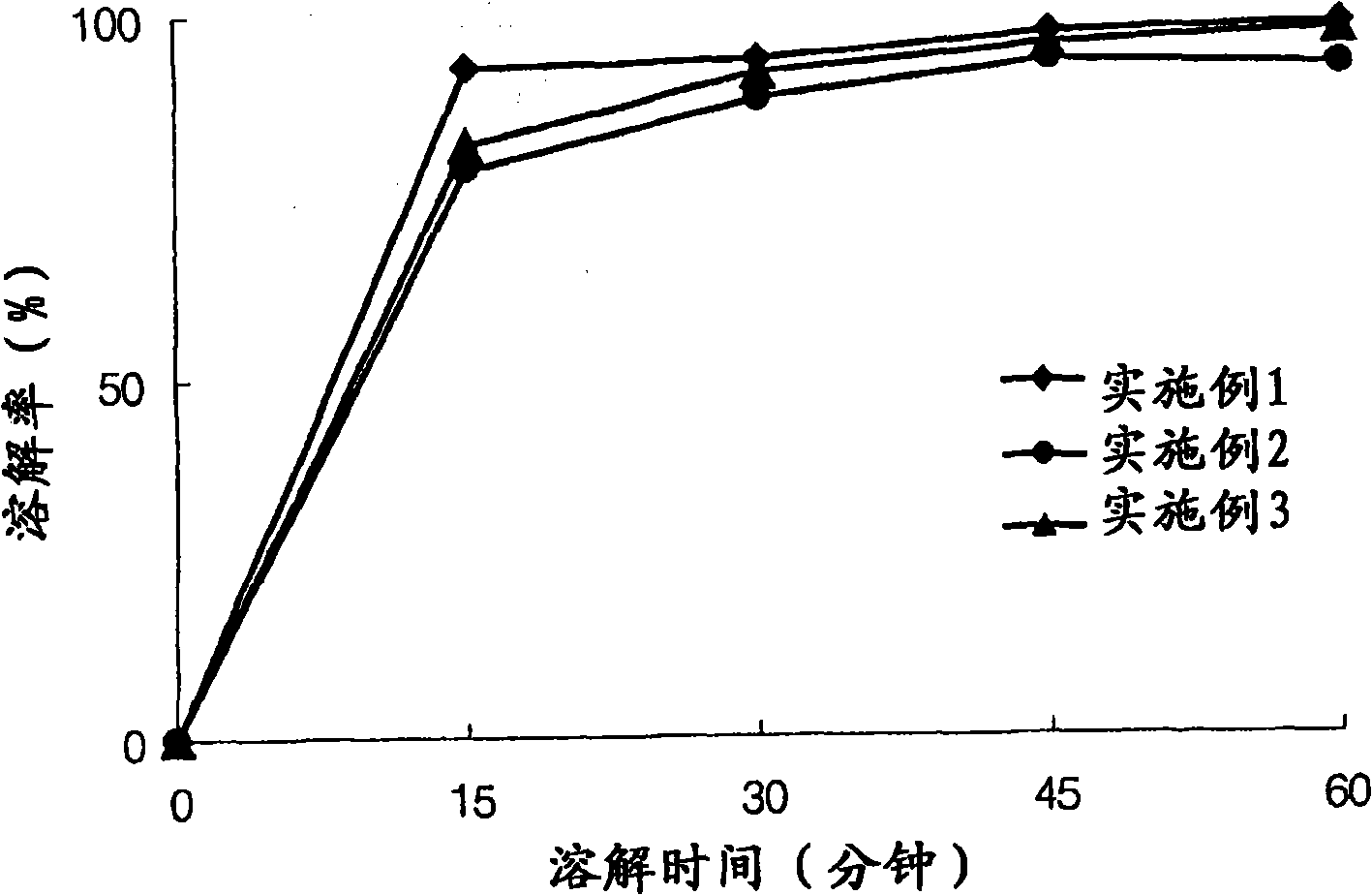
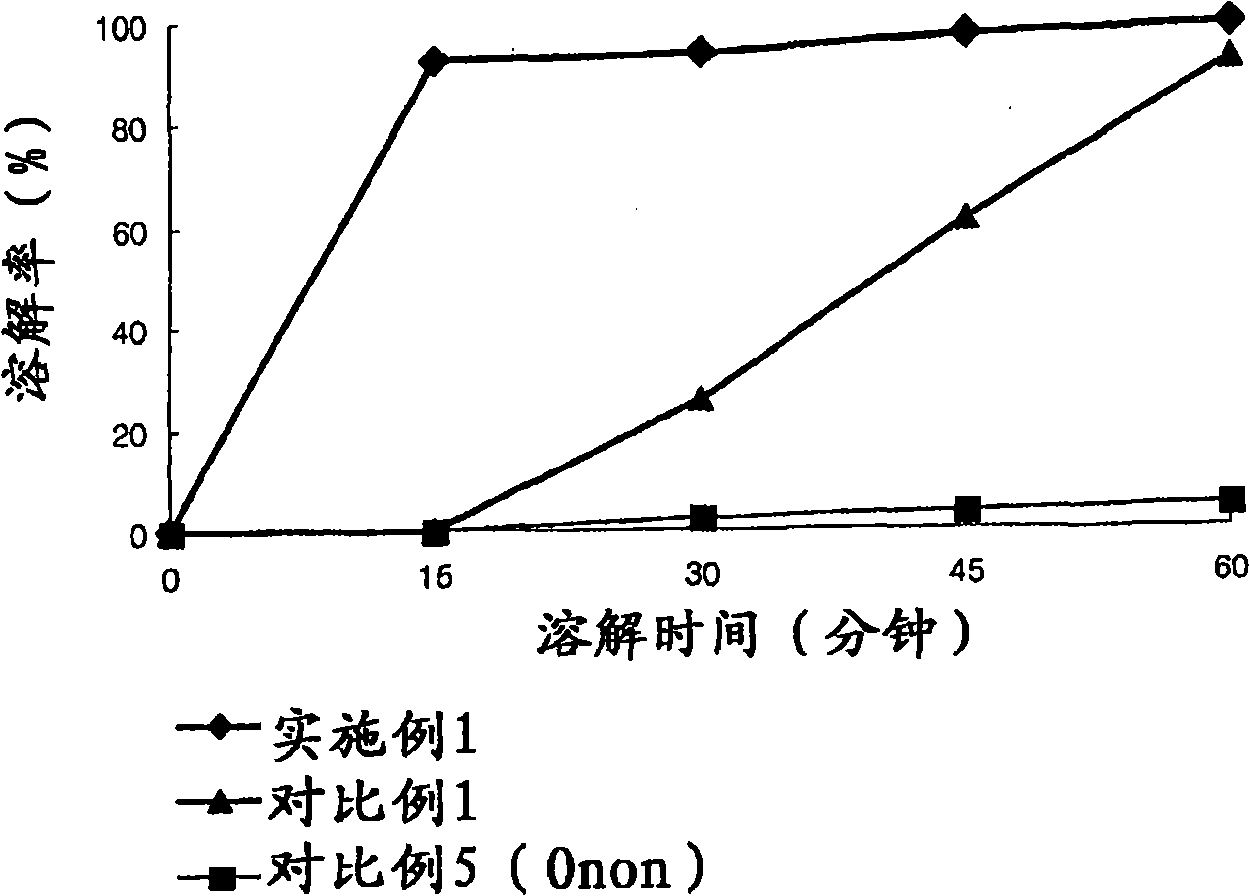
[0046] Experimental example 1: Measuring the dissolution rate over time
[0047] The commodity Onon in embodiment 1-15 and comparative example 1-4 and comparative example 5 Capsules prepared in Capsules were subjected to a dissolution test at a temperature of 37.5° C. in a dissolution medium of pH 6.8 with stirring at a speed of 50 rpm for 60 minutes. The time-dependent solubility was analyzed using HPLC, and the dissolution rate was calculated using Mathematical Formula 1 below, as provided in Table 4.
[0048] Mathematical formula 1
[0049]
[0050] Wherein C represents the weight (mg) of pranlukast in each capsule.
[0051] Table 4
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com