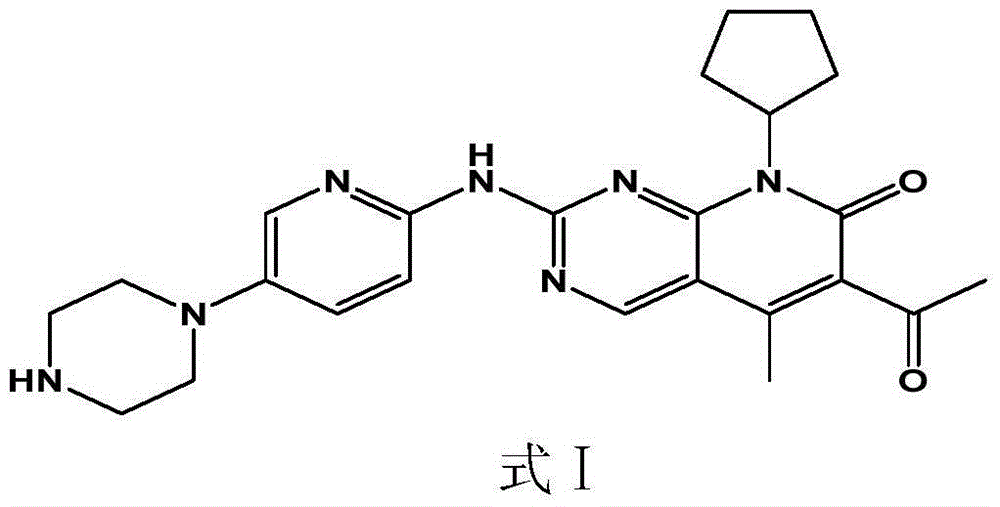
Pharmaceutical preparation containing cyclin inhibitor, and preparation method thereof
A drug and composition technology, applied in the field of pharmaceutical preparations and preparations of cyclin inhibitors, can solve the problems of high incidence and slow progress of the disease, and achieve the effects of excellent dissolution behavior and good bioavailability in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1.1 Unit prescription composition
[0046]
[0047]
[0048] Note: " / " means not added.
[0049] 1.2 Preparation
[0050] Put the above-mentioned 1,000 tablets of the formula I compound and other excipients except magnesium stearate into the hopper mixer and mix evenly, add a wetting agent to moisten the granules, dry on a fluidized bed at 45°C for 10 minutes, and sieve the granules with a size of 1.0 mm. Add magnesium stearate and mix for 10 minutes, control the content in the middle, and fill the capsules. Or compressed into tablets.
[0051] 1.3 Dissolution data
[0052]
Embodiment 2
[0054] 2.1 Composition of unit prescription
[0055]
Prescription 9
Prescription 10
Prescription 11
Prescription 12
Formula I compound
75
75
100
125
38.7
10.8
93.9
117.3
54.3
18.8
49.7
65.7
/
/
/
17.5
Povidone
10.5
3.5
14
/
/
10.5
14
17.5
21
/
/
/
2.1
/
2.8
/
2.1
0.7
2.8
3.5
2.1
0.7
2.8
3.5
weight
210
120
280
350
[0057] Note: " / " means not added.
[0058] 2.2 Preparation
[0059] Put the above 1000 tablets of formula Ⅰ compound and other excipients except magnesium stearate into the hopper mixer for mixing, use a roller p...
Embodiment 3
[0063] 3.1 Unit prescription composition
[0064]
Prescription 13
Prescription 14
Prescription 15
Formula I compound
75
75
75
71.8
/
50
35.9
25.8
15.7
10.5
6.0
9.0
10.5
6.0
5.0
Crospovidone
/
3.0
4.0
2.1
/
2.1
2.1
2.1
2.1
Magnesium stearate
2.1
2.1
2.1
weight
210
120
165
[0065] Note: " / " means not added.
[0066] 3.2 Preparation
[0067] Put the above 1000 tablets of the formula I compound and excipients into the hopper mixer and mix evenly, control the content in the middle, and fill the capsules. Or compressed into tablets.
[0068] 3.3 Dissolution data
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com