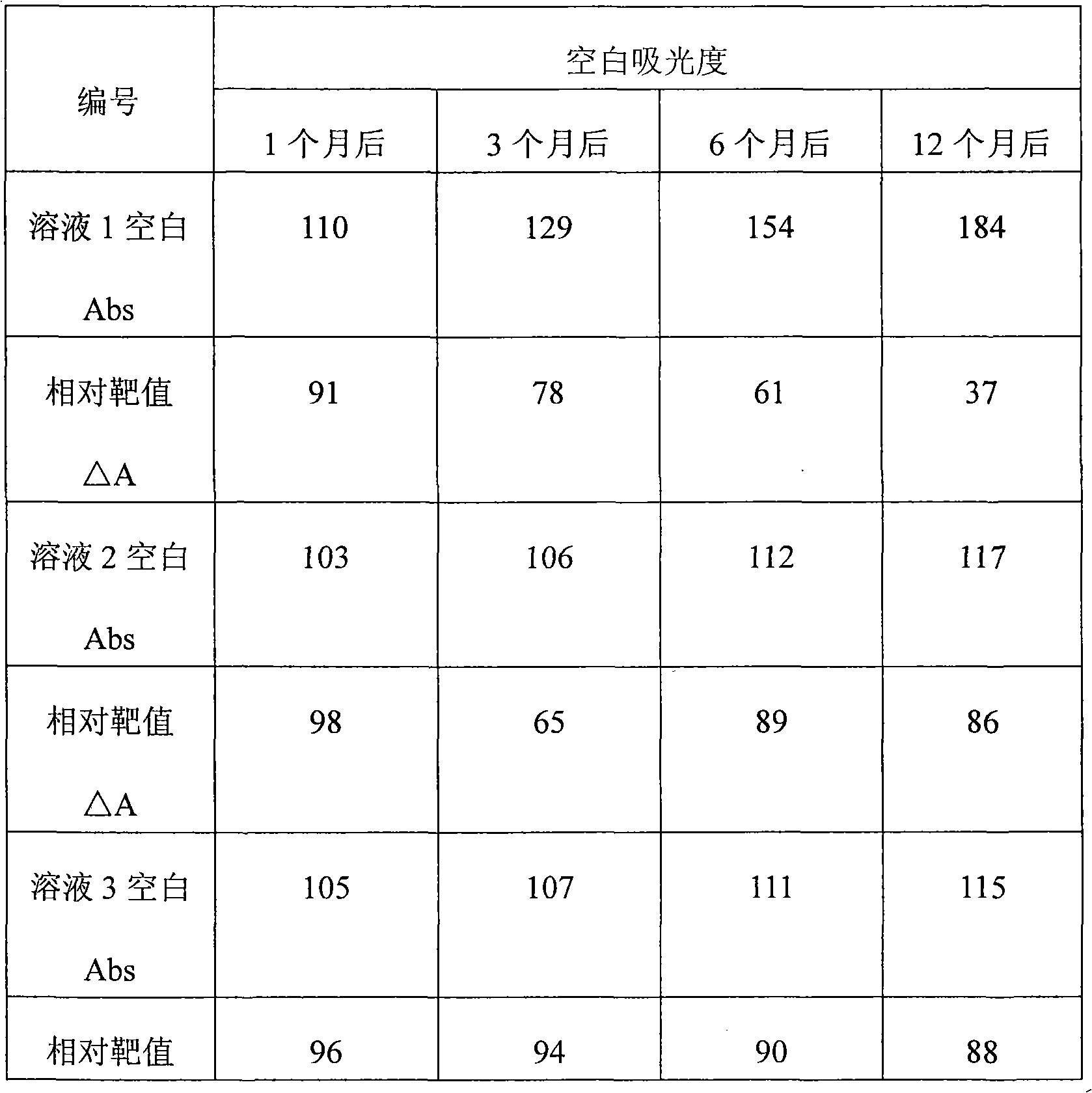
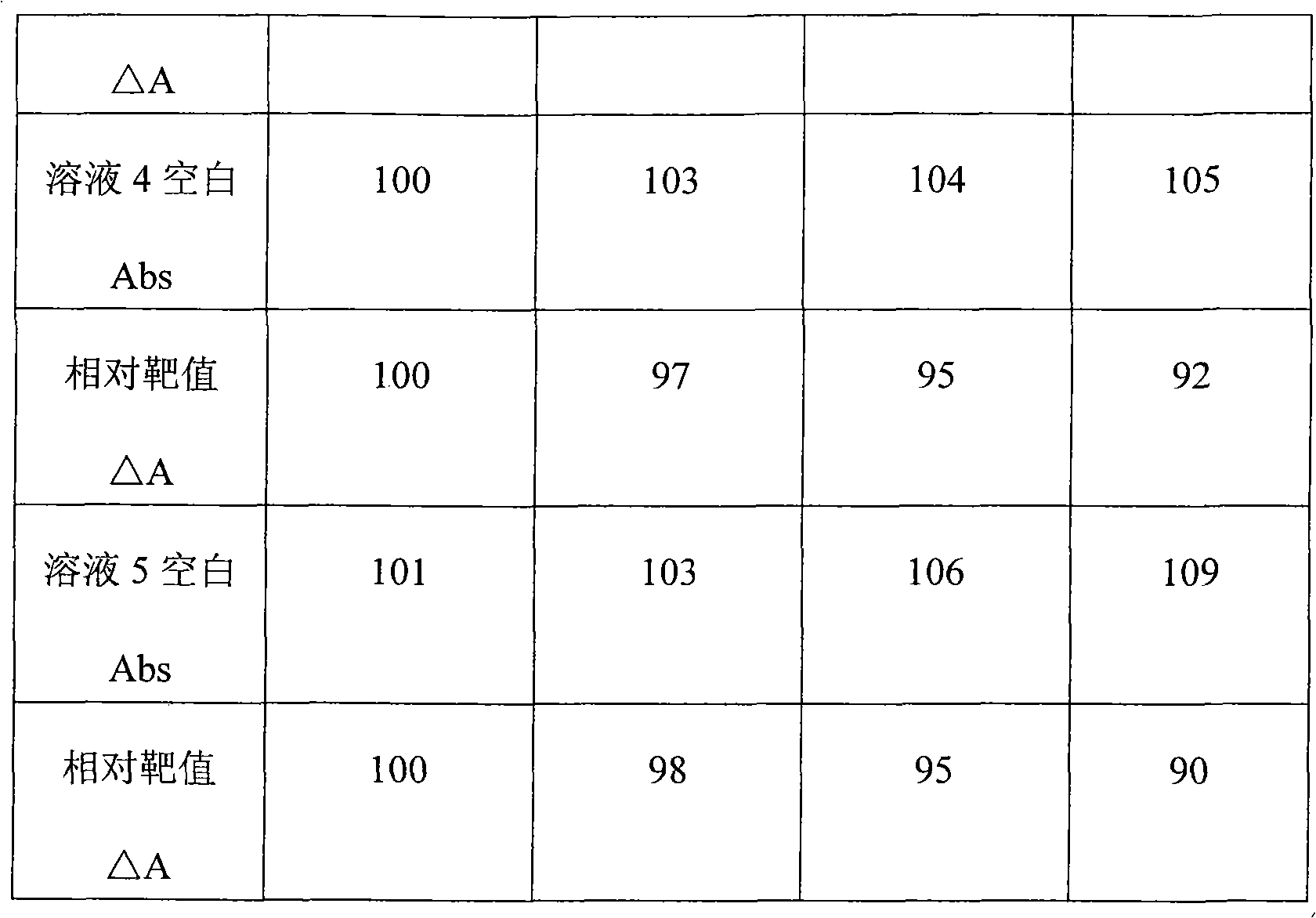
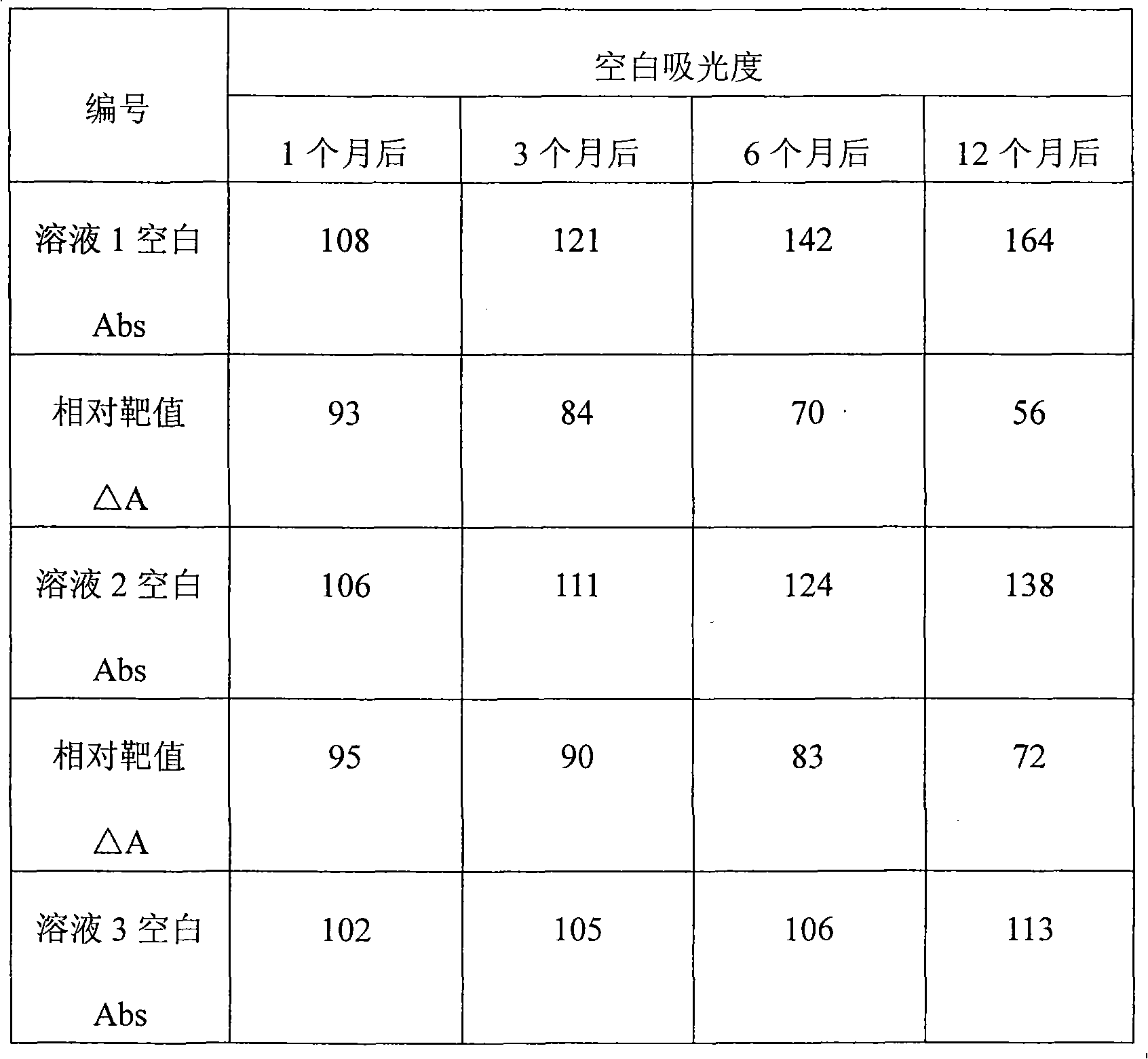
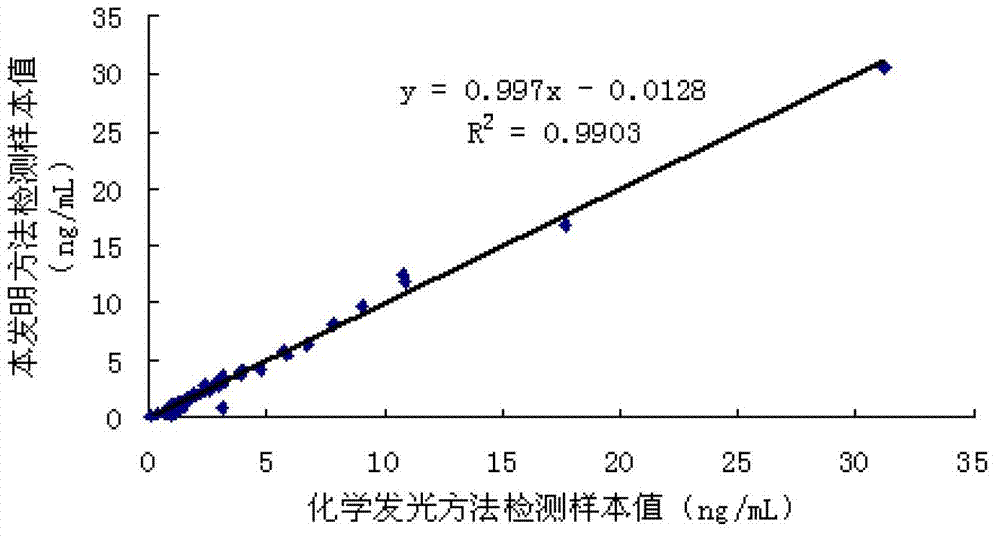
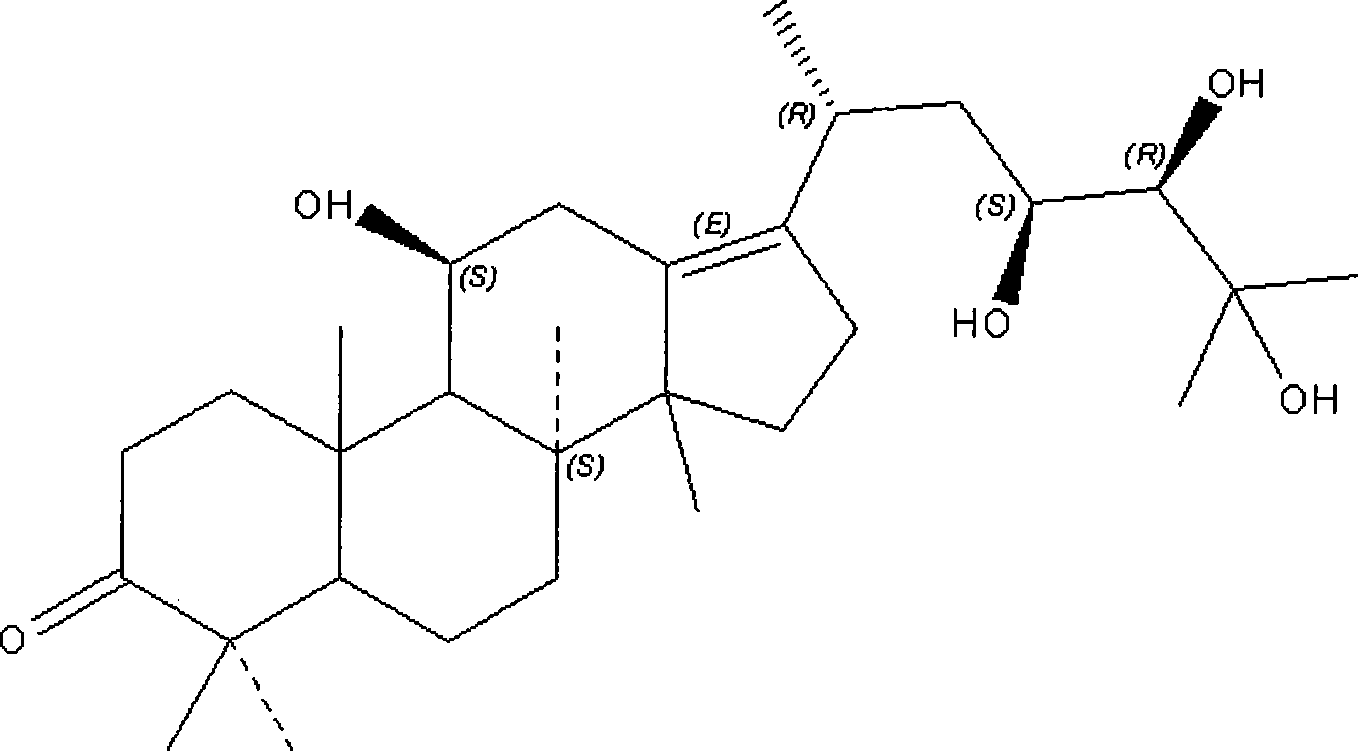
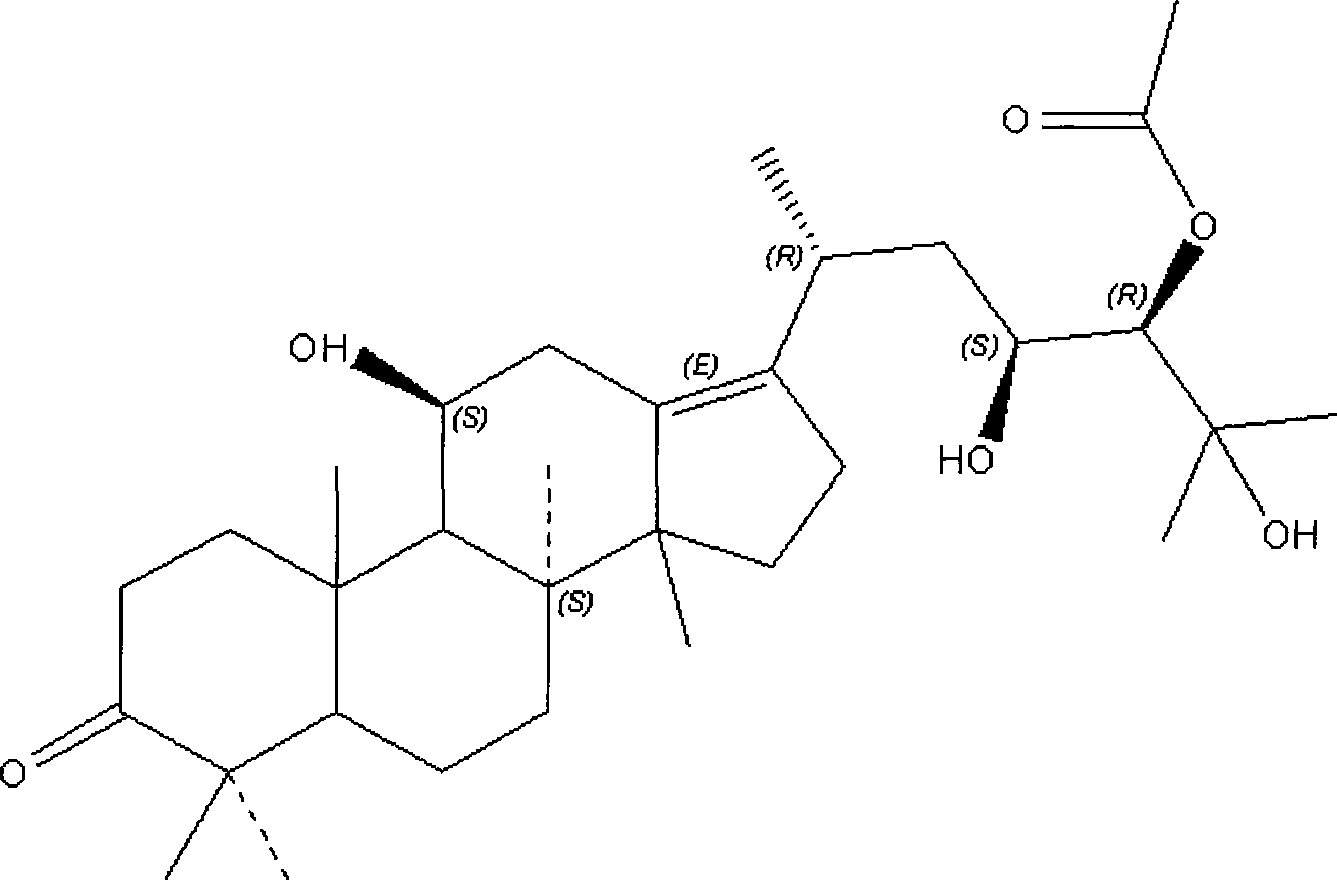
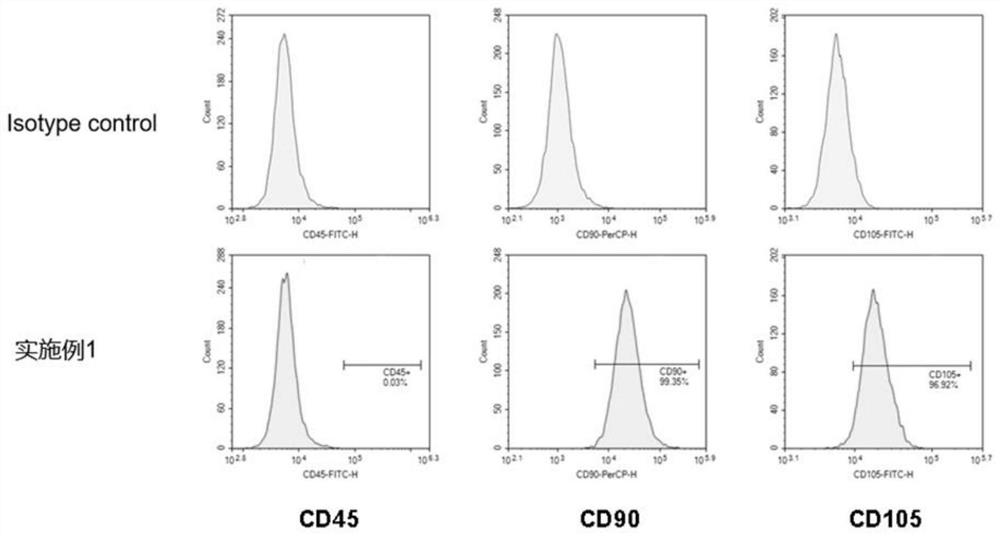
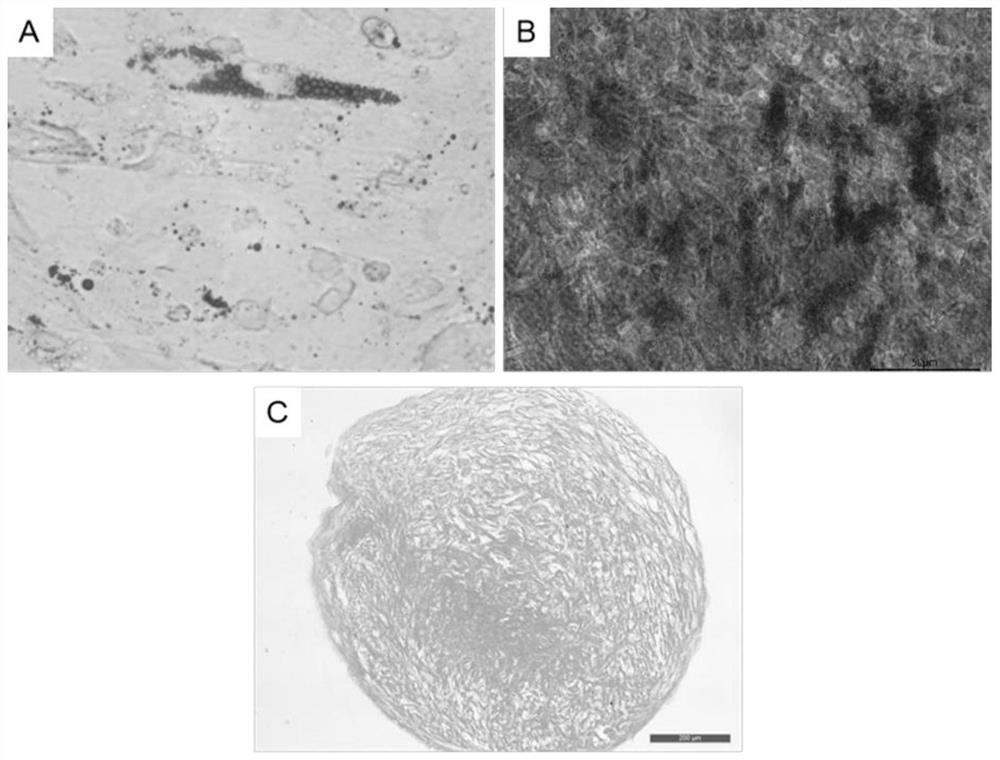
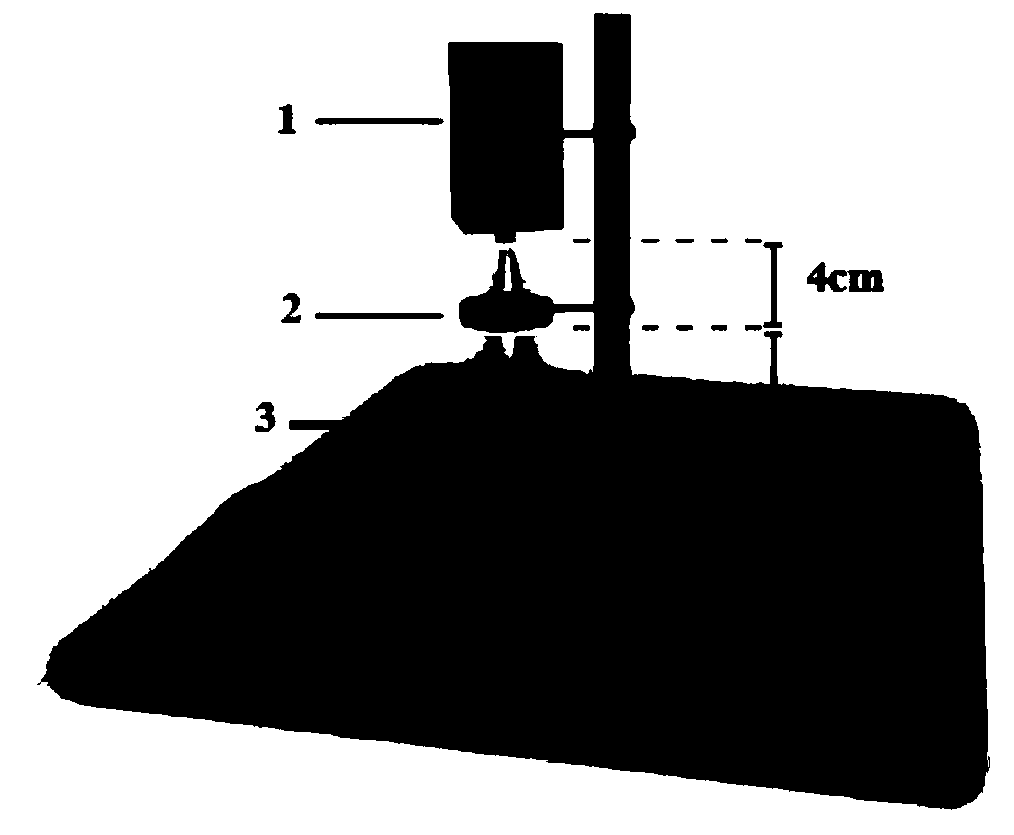
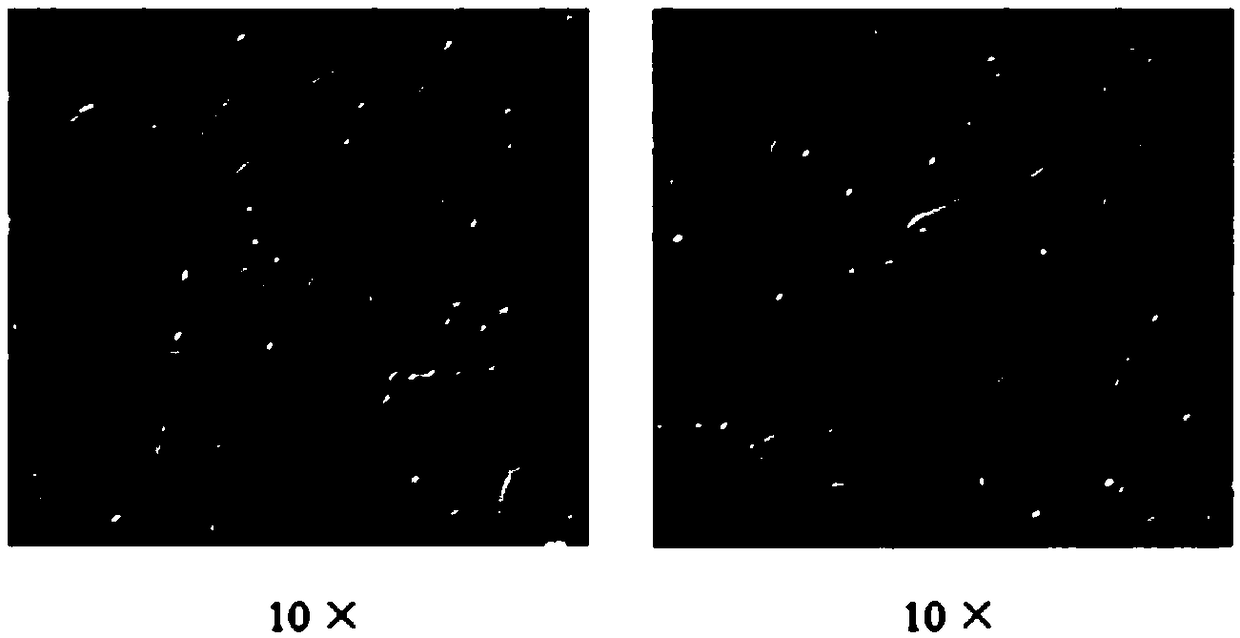
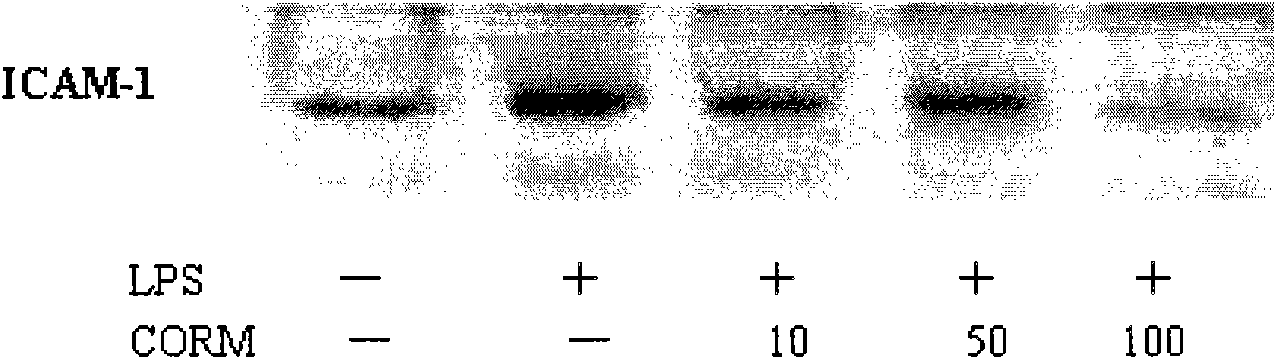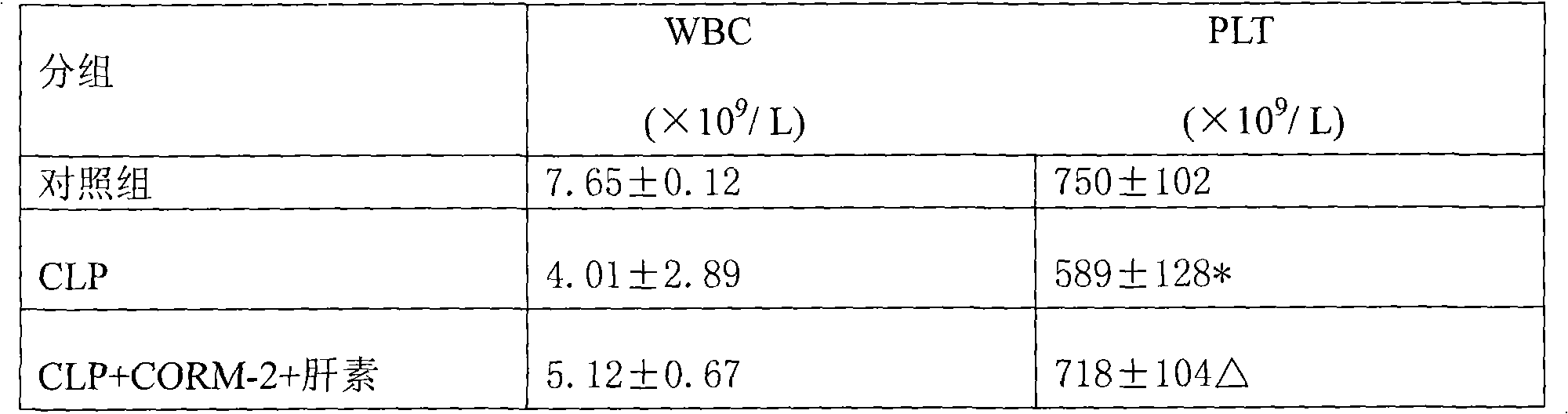Patents
Literature
57results about How to "Meet the needs of clinical application" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Processable aluminium oxide base composite ceramic material and preparation method thereof
The invention discloses a processable aluminium oxide base composite ceramic material, which comprises the following raw materials in parts by mass: 60-88 parts of Al203, 5-15 parts of MgO, 5-15 parts of TiO2, 1-5 parts of SiO2 and 1-5 parts of CaO. The preparation method thereof comprises the following steps: mixing Cao powder and SiO powder in a proportion of 1:1 to prepare a sintering assistant; premixing MgO powder and TiO2 powder, then mixing with Al203 powder and the sintering assistant, ball-milling, drying the powder by ball-milling, and hot pressed sintering and molding at vacuum atmosphere. The invention overcomes the shortages that the aluminium oxide ceramic has higher hardness, and is difficult to process, produces a compound ceramic material with certain processability, and is especially applicable to a full ceramic reparation material in dentistry.
Owner:SHIJIAZHUANG TIEDAO UNIV
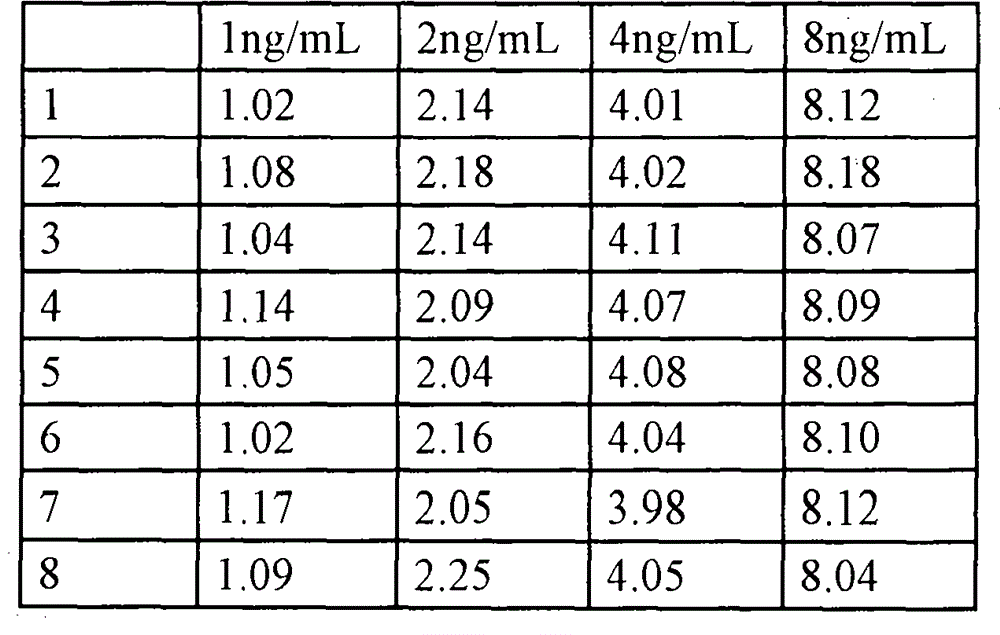
Method for improving stability of latex suspension liquid
The invention provides a method for improving the stability of latex suspension liquid, comprising the following steps of adding nonionic surfactant and substances which can enhance the condition of surface charging of particles in reagent solution or regulating pH of the reagent solution. The invention can make a biological fluid sample in a stable state when quantitatively determining the biological fluid sample and analyzing the concentration of an analyte in the sample and can achieve better repeatability of a measurement result of a clinical diagnosis reagent without various problems of precipitate at the bottom of the reagent, gap elevating, sensitivity lowering and the like after being stored for a certain period.
Owner:AILEX TECH GRP CO LTD +1
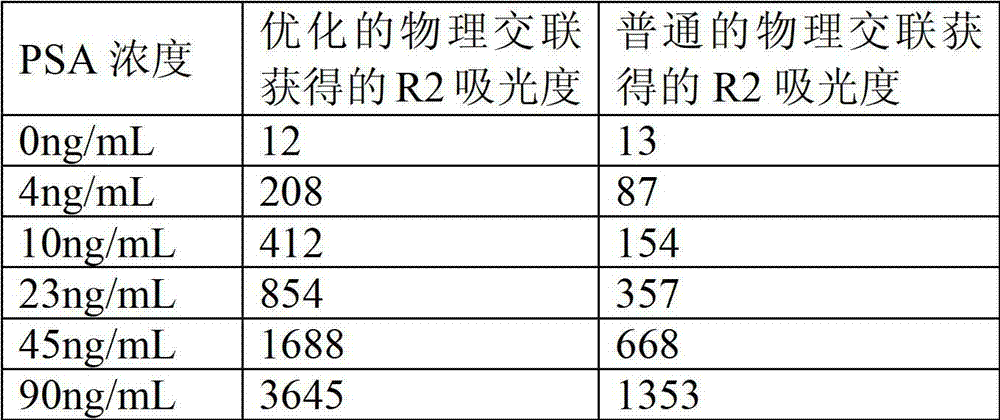
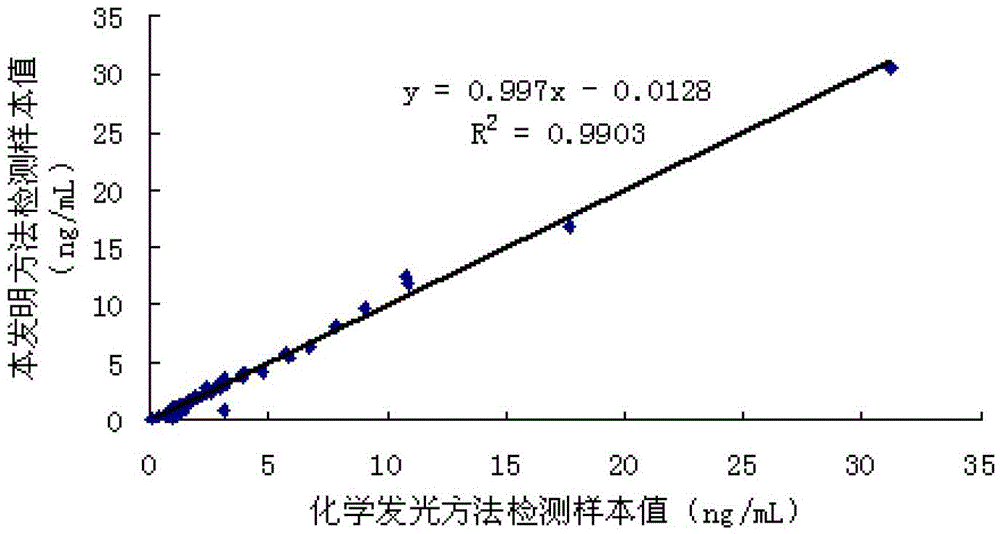
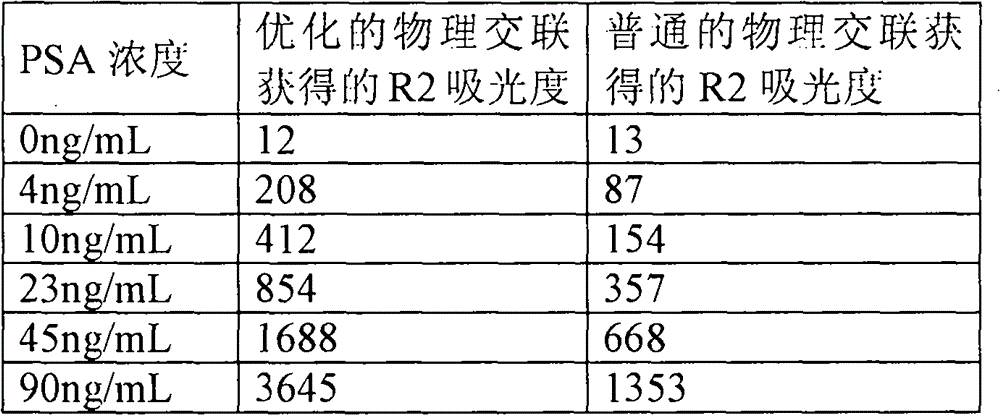
Preparation method of latex particles coated with prostate specific antigen-antibody and PSA enhanced turbidimetric immunophelometry kit
ActiveCN102901810AImprove featuresHigh sensitivityMaterial analysis by observing effect on chemical indicatorAntigenPSA Antibody
The invention relates to a preparation method of latex particles coated with a prostate specific antigen-antibody (PSA) and a PSA-enhanced turbidimetric immunophelometry kit. The latex particles coated with the PSA antibody are prepared by bonding the PSA antibody with latex particles by an optimized physical adsorption method. According to the invention, the kit utilizes the PSA antibody bonded onto the surface of the latex particles and the PSA in the human blood sample to conduct immunoreaction to form the turbidity, and the kit detects the content of the prostate specific antigen-antibody by the increase of the turbidity.
Owner:BEIJING STRONG BIOTECH INC
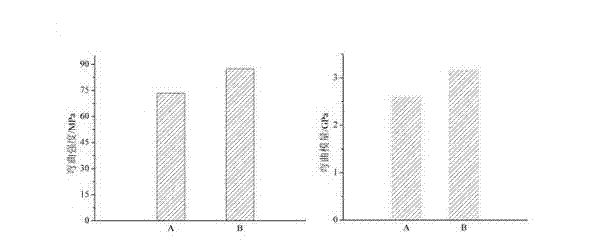
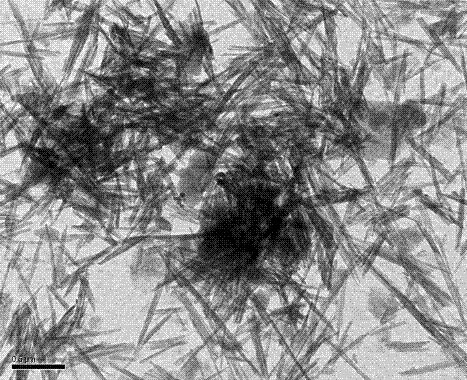
Antibacterial type inorganic whisker functional composite as well as preparation method and application thereof
InactiveCN103040631AEasy to prepareSimple operation processImpression capsDentistry preparationsPrepolymerPhotoinitiator
The invention relates to an antibacterial type inorganic whisker functional composite which is a composite consisting of antibacterial type inorganic whisker particles and acrylic polymer. The antibacterial type inorganic whisker functional composite has an application of uniformly mixing the antibacterial type inorganic whisker functional composite with an acrylic monomer mixture, surface-modified silica, a photoinitiator and a coinitiator to obtain uncured resin plaster, and curing the uncured resin plaster by a visible light or an ultraviolet light to obtain antibacterial type dental prosthesis composite resin. The antibacterial type inorganic whisker functional composite can carry out reaction through surface modification and surface covering to produce prepolymer, so that the organic-inorganic interface bonding force in the composite resin is effectively improved, the mechanical properties of the composite are improved, the bending strength is 87.4-135.3 MPa, the bending modulus is 3.16-5.82 GPa, and the mass percentage of a dissolution value is 0.02-0.08 percent per 30 days; in addition, the antibacterial type inorganic whisker functional composite has excellent antibacterial property and is safe and nontoxic; and with the adoption of the antibacterial type inorganic whisker functional composite, secondary dental caries can be effectively avoided, and the needs of clinical application are well satisfied.
Owner:CHANGZHOU HIGH TECH RES INST OF NANJING UNIV
Composite nose prosthesis
The composite type nasal prosthesis is characterized by that the back of nose of L-type or lance-type prosthesis made of solid silicone rubber material has an enlongated slot, a long strip body whichis made of bulky teflon material and correspondent to the enlogated slot. The present invention adopts the bulky teflon material with excellent compatibility with human body tissue in the key position-back of nose and apex of nose, after said material is implanted into human body, the human self-body tissue cell can be grown into bulky teflon material, so that it can effectively prevent the material from producing rejection, motion and ulceration, etc. at the same time it also can prevent light reflex, and can make the plasticized face form more natural and lifelike. The solid silicone rubberbose as base seat has a certain hardness, its cutting process is simple, and its implantation is more convenient, and its cost is low.
Owner:叶明
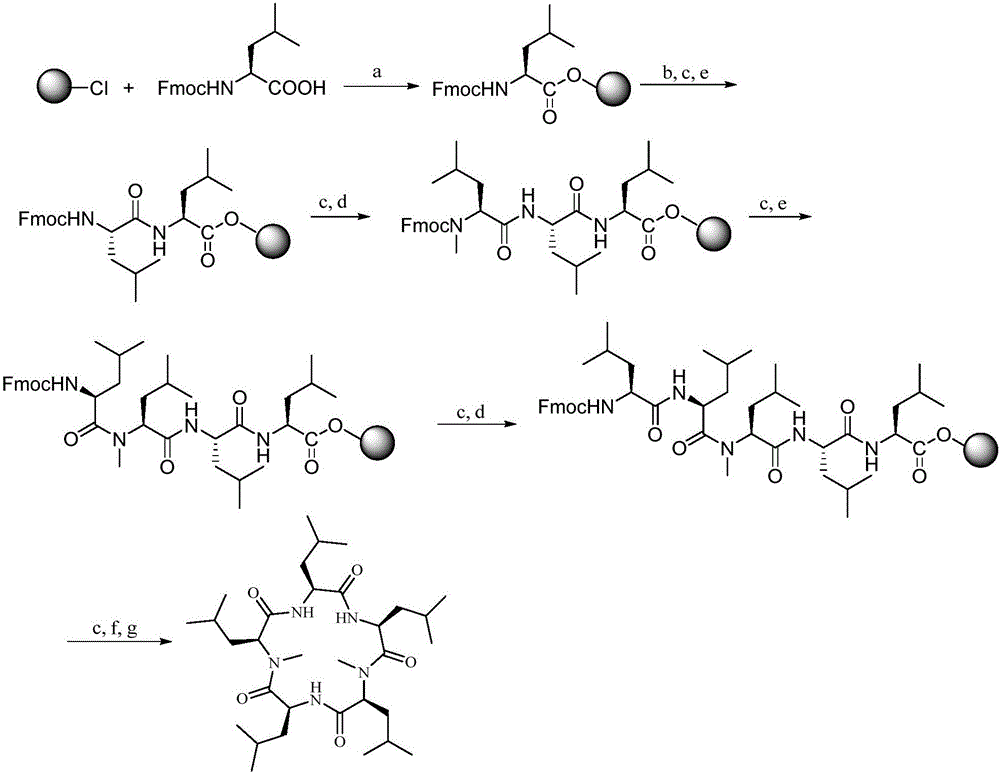
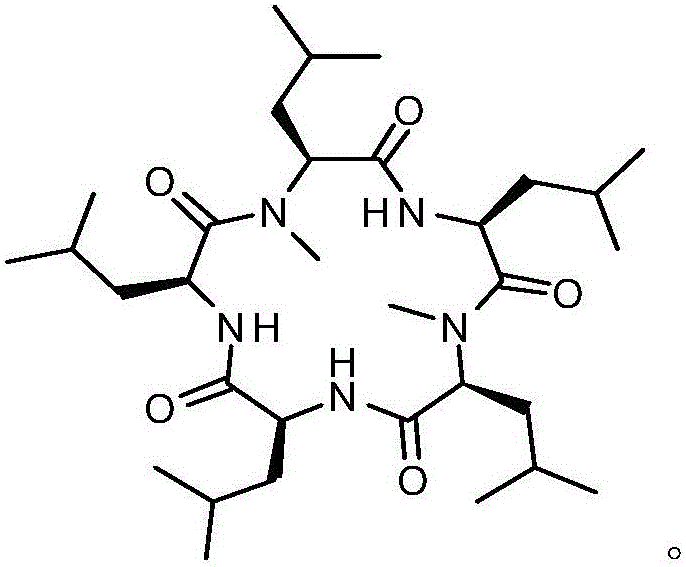
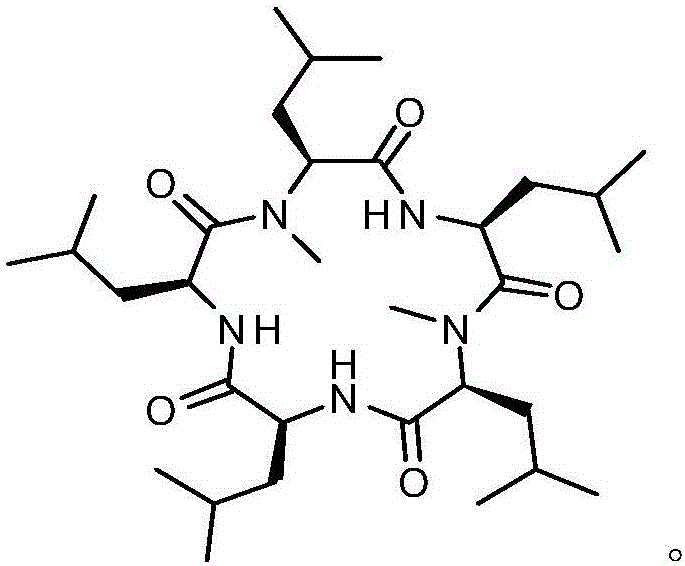
Synthesis method of cyclic pentapeptide
ActiveCN105777871AHigh purityReduce racemizationPeptide preparation methodsSynthesis methodsDrug biological activity
The invention discloses a synthesis method of cyclic pentapeptide. According to the synthesis method, the 'solid-liquid combined production' strategy is adopted, a proper condensation agent is used for a condensation reaction, linear pentapeptides are prepared respectively through a proper process, the cyclization site is located between N-methyl-leucine and leucine, and the efficiency of linear peptide preparation is effectively improved.The proper condensation agent is used for the condensation reaction, racemization in amido bond formation can be effectively reduced, therefore, the purity of a target product can be effectively improved, reaching 99% or above, the requirements of biological activity tests and clinical application can be fully met, and the synthesis method of cyclic pentapeptide has high medical value. The cyclization agent PyBOP is used for a cyclization reaction, the cyclization yield can be effectively increased and reach up to 84.2%.The synthesis method does not require complex purification by column chromatography, redundant amino acids can be simply washed away, the synthesis process is simple, the cost of raw materials is low, reaction conditions are mild, the purity is high, industrialization is easy, and the synthesis method has broad market prospects.
Owner:JINAN UNIVERSITY
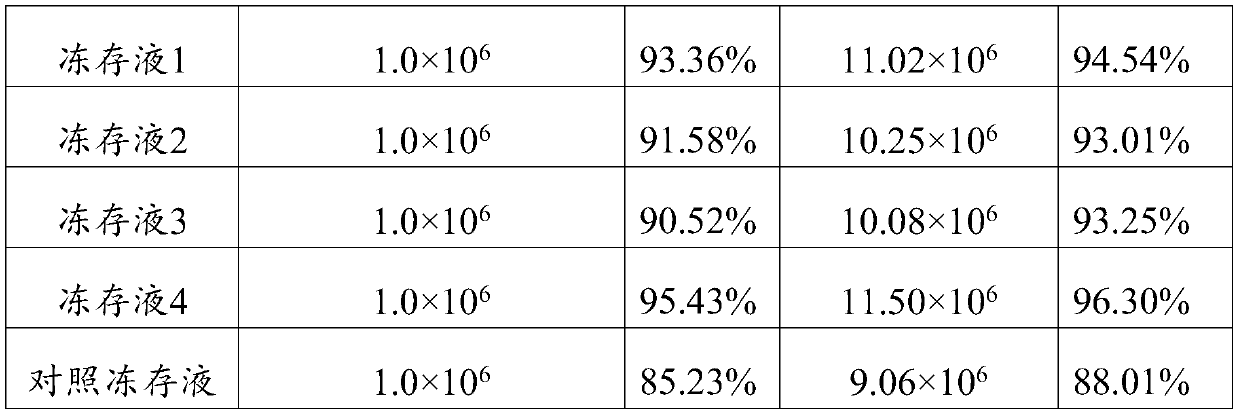
Method for ultralow-temperature cryopreservation and resuscitation of mesenchymal stem cells
InactiveCN109699634AMeet the needs of clinical applicationReduce entryDead animal preservationSkeletal/connective tissue cellsGas phaseMotility
The invention provides a serum-free cryopreservation solution for mesenchymal stem cells and a method for ultralow-temperature cryopreservation and resuscitation of the mesenchymal stem cells by usingthe cryopreservation solution. Compared with a fetal calf serum containing cryopreservation solution and a cryopreservation and resuscitation method employing the fetal calf serum containing cryopreservation solution in the prior art, the serum-free cryopreservation solution and the method have the advantages that through subjecting the mesenchymal stem cells to cryopreservation by using the serum-free cryopreservation solution provided by the invention, fetal calf serum is not added during cryopreservation, and thus, risk possibly caused by introduction of heterogeneous proteins is avoided;and through adopting sealing operations and a programmed cooling box and storing cells by a gas-phase liquid-nitrogen tank, the entry of liquid nitrogen during cryopreservation is effectively reducedwhile a cryopreservation effect is ensured, so that risk of pollution during cryopreservation and tube burst during resuscitation is lowered, the motility rate of the mesenchymal stem cells after resuscitation reaches 90% or more finally, and the requirements of the mesenchymal stem cells in clinical application can be met.
Owner:和携科技有限公司
Medical porous alumina based ceramic composite material
InactiveCN101757683AImprove mechanical propertiesFacilitate depositionProsthesisCeramic compositeNitrogen gas
The invention belongs to the field of biological ceramic materials, and relates to a medical porous alumina based ceramic composite material. Firstly, 1-8% of calcium fluoride, 90-98% of alumina and 1-5% of diopside are mixed according to the mass percentage, and then the calcium fluoride, the alumina and the diopside are milled by balls to prepare mixed powder; and then the mixed powder material is placed into a graphite mould to be molded and pressed, hot-pressed and sintered in the argon or nitrogen atmosphere, wherein the sintering temperature is 1400 DEG C, the pressuring temperature is 1320 DEG C, the sintering pressure is 30 MPa and the heat preservation and pressure maintaining are carried out for 30 minutes at the sintering temperature. The composite material not only has good mechanical property and bioactivity, but also is distributed with micropores on the surface and is more beneficial to the growth of bone tissues. Simultaneously, the composite material has low manufacture cost and better application prospect in terms of the restoration, replacement and the like of bones of the human body.
Owner:SHANDONG UNIV
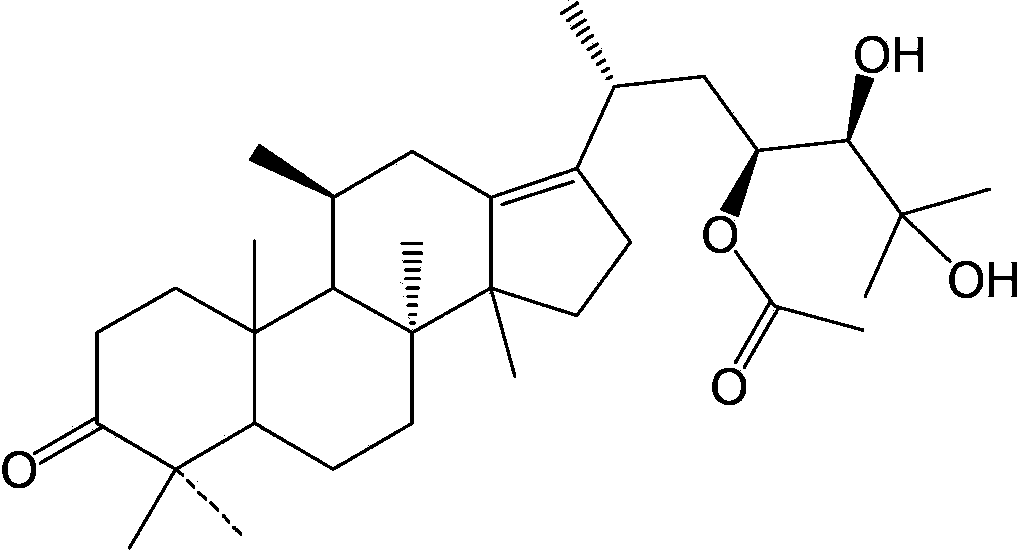
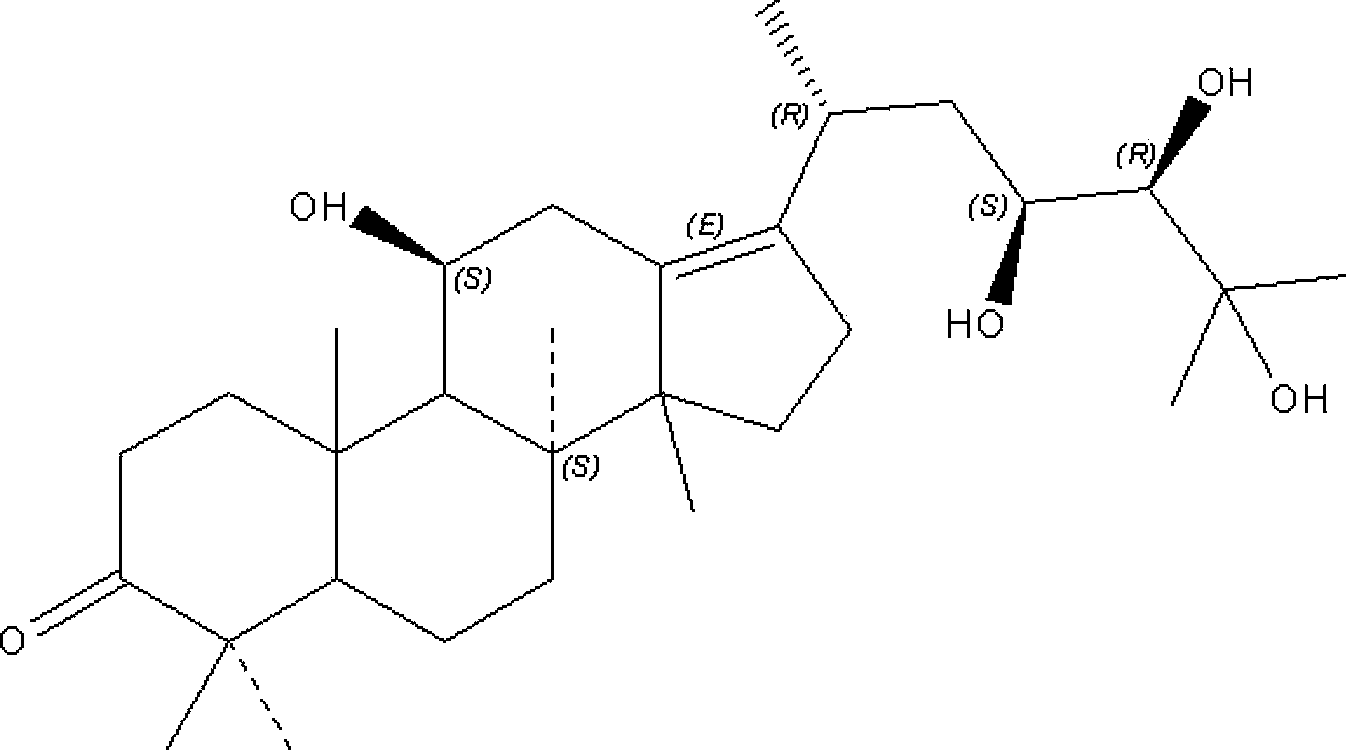
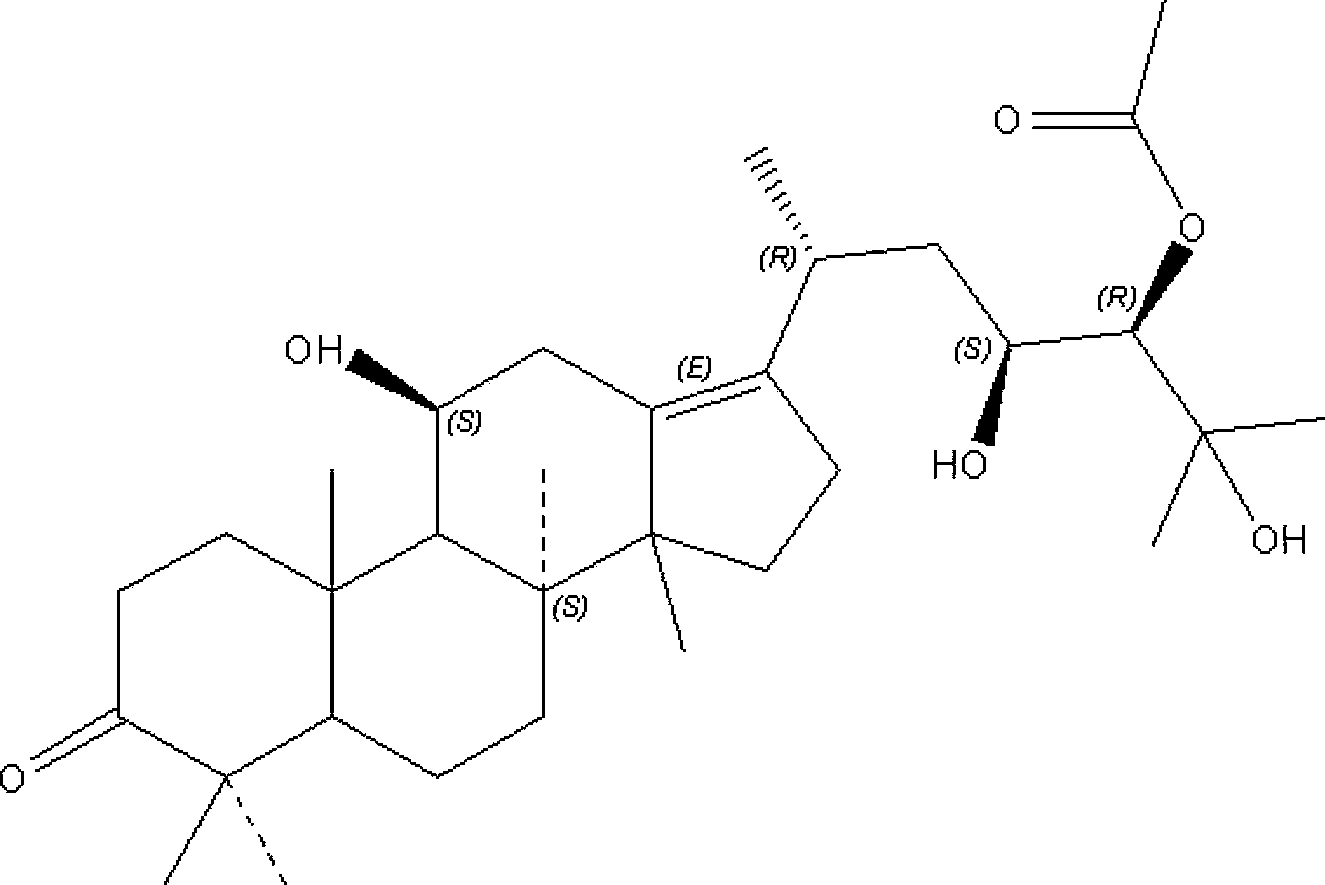
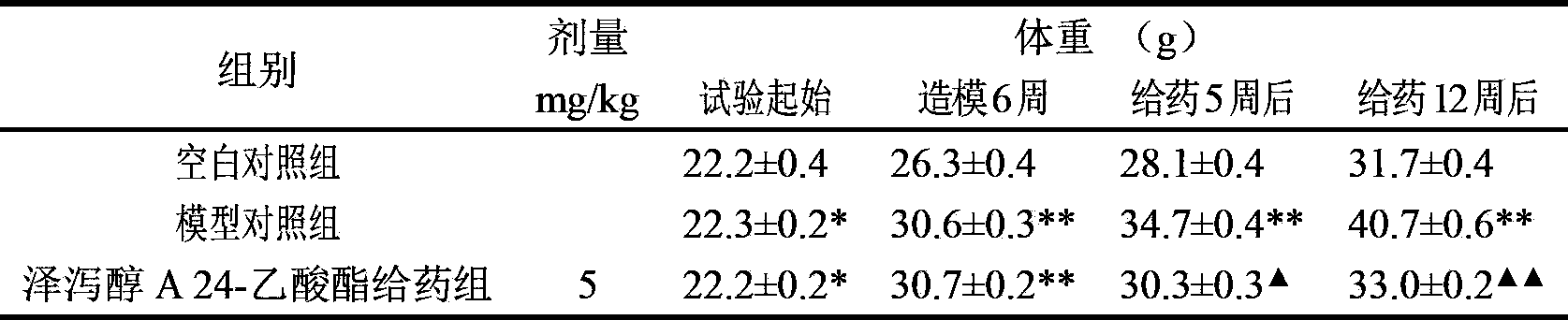
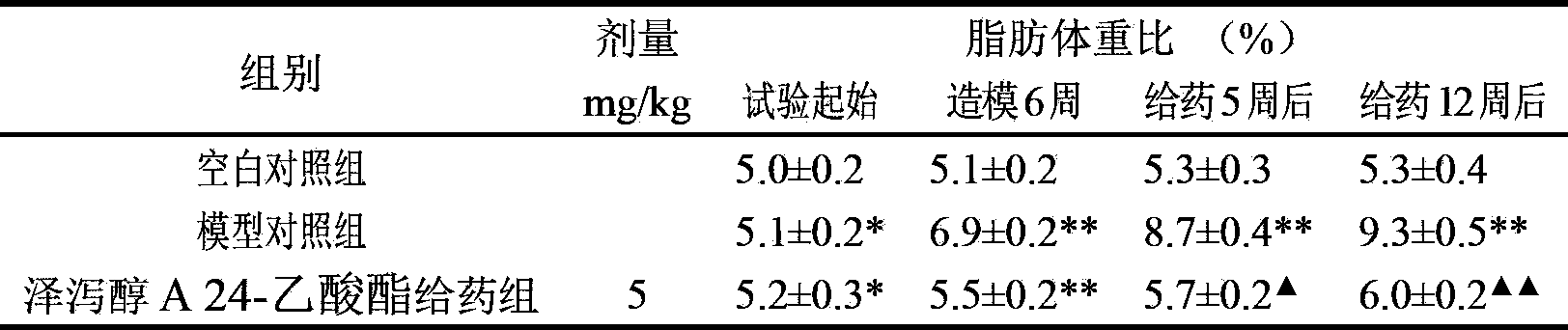
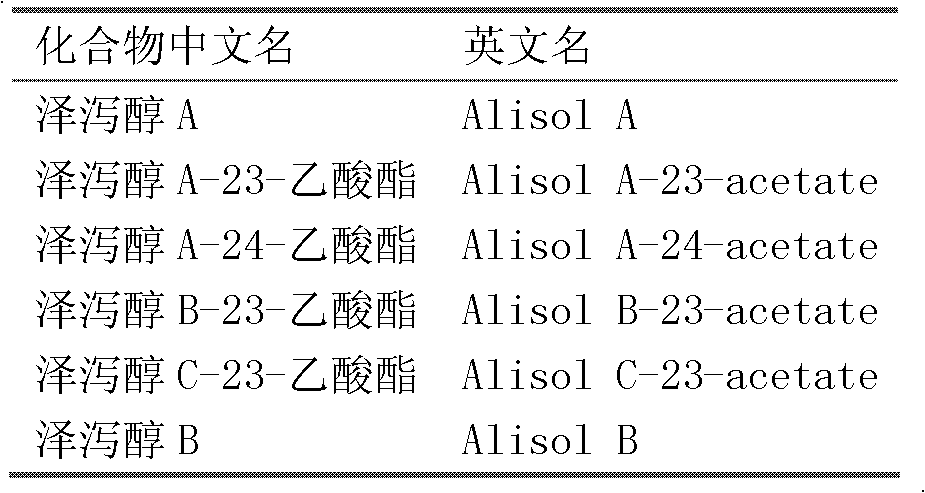
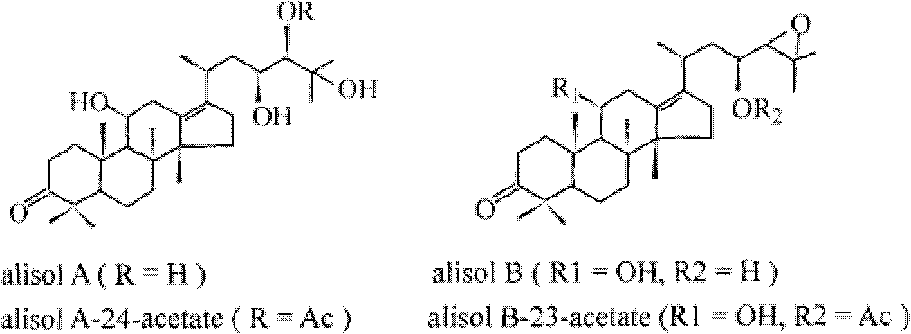
Composition containing alisol A and alisol A 24-acetic ester and use
ActiveCN101411711ALow toxicityNo damageOrganic active ingredientsMetabolism disorderFatty liverSide effect
The invention provides a composition consisting of alisol A and alisol A24-acetic ester and application thereof, wherein weight ratio of the composition consisting of the alisol A and the alisol A24-acetic ester is between 1: 0.02 and 1: 50. The invention provides a medicine for treating hyperlipidemia diseases, atherosclerosis diseases or fatty liver diseases by modern medical theory so as to satisfy needs for clinical application. The invention has the advantages that people taking the composition consisting of the alisol A and the alisol A24-acetic ester for a long time do not have abnormal rise of liver transaminase, intrahepatic fat deposit, or increase of liver volume and / or weight. The creativity of the invention lies in that through a systematically study of the specific mixture ratio of the alisol A and the alisol A24-acetic ester, the curative effect of decreasing blood-fat, resisting atherosclerosis and treating fatty liver can be improved, and toxic and side effect can be reduced.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
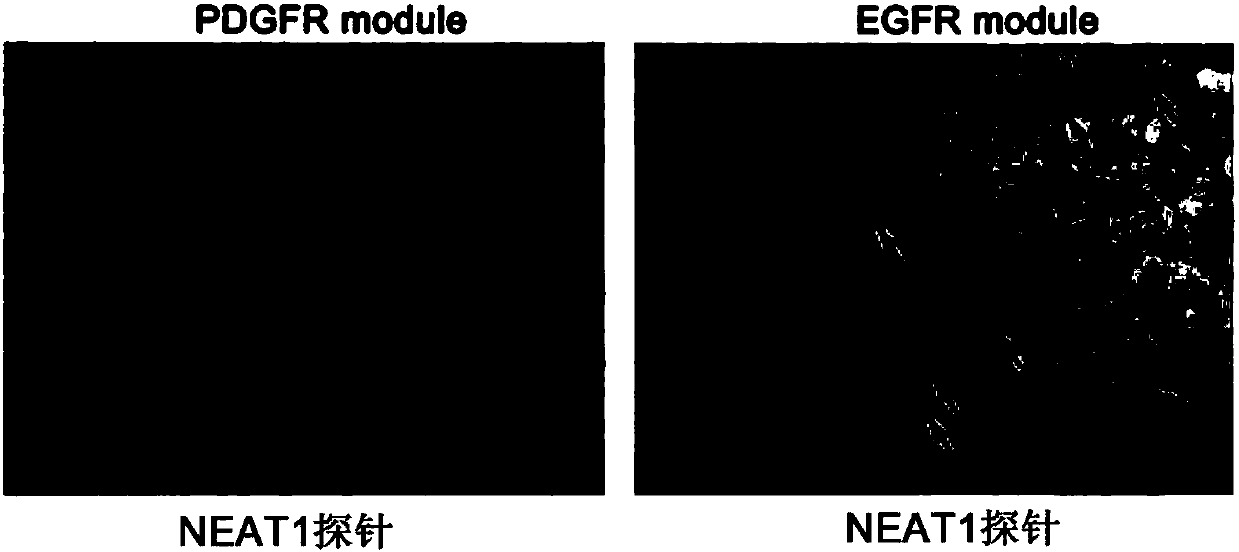
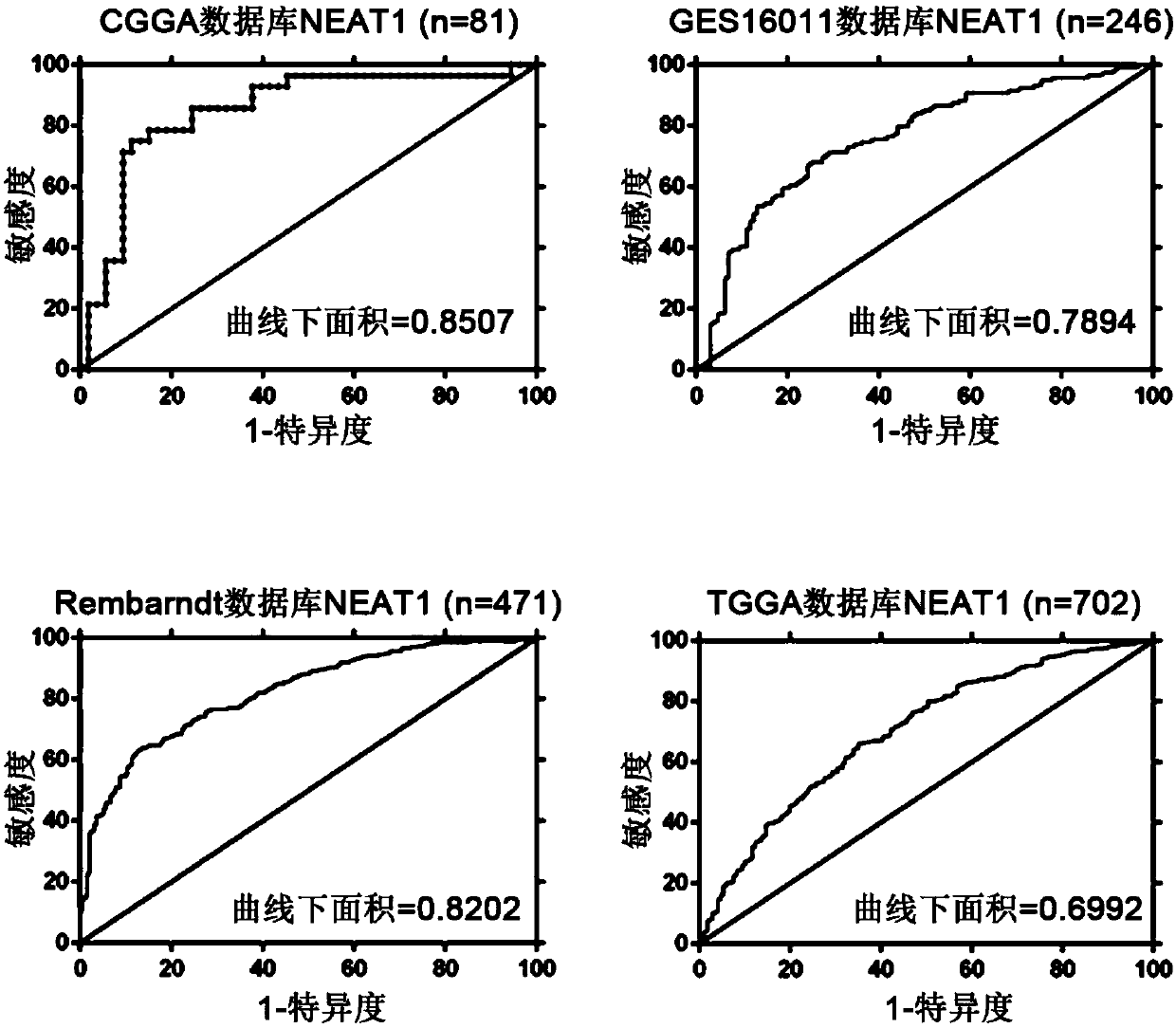
Application of lncRNA-NEAT1 as molecular pathological diagnosis marker in preparation of glioma diagnosis reagent
InactiveCN107779508ALow costHigh sensitivityMicrobiological testing/measurementDiagnosis methodsPcr method
The invention discloses application of lncRNA-NEAT1 as a molecular pathological diagnosis marker in preparation of a glioma diagnosis reagent. According to the invention, a glioma public database is utilized for screening lncRNA related to EGFR module and PDGFR module type gliomas, and detection on tumor tissues of a glioma patient finds that lncRNA-NEAT1 can be detected in the tumor tissues, andthe lncRNA-NEAT1 content of the EGFR module tumor tissues is obviously higher than that of PDGFR module, so that the marker can be used for distinguishing the EGFR module type glioma and the PDGFR module type glioma. Besides, the invention also provides a glioma diagnosis kit and establishes a glioma diagnosis method. The method is carried out by virtue of a PCR process, has the advantages of lowcost, short time and high sensitivity compared with the traditional method and can meet large-scale experiment and clinical application needs.
Owner:HARBIN MEDICAL UNIVERSITY
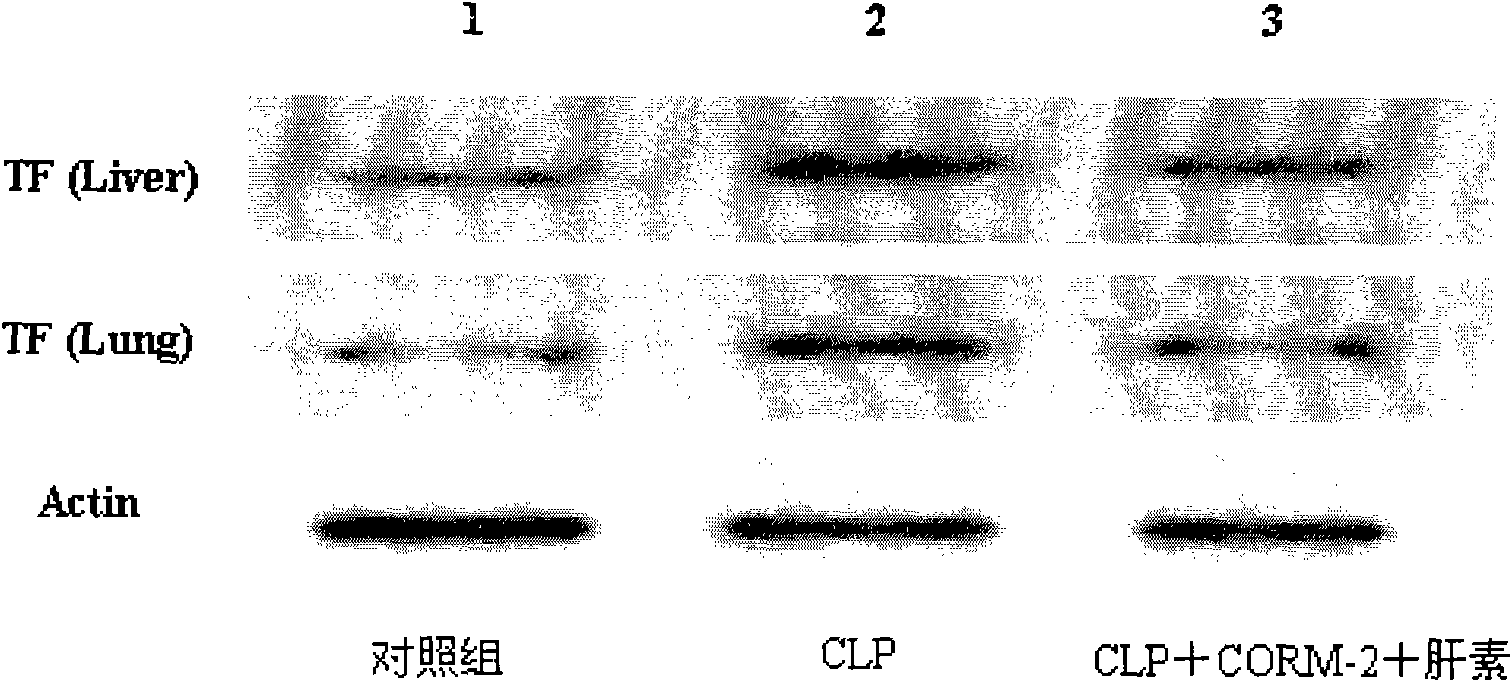
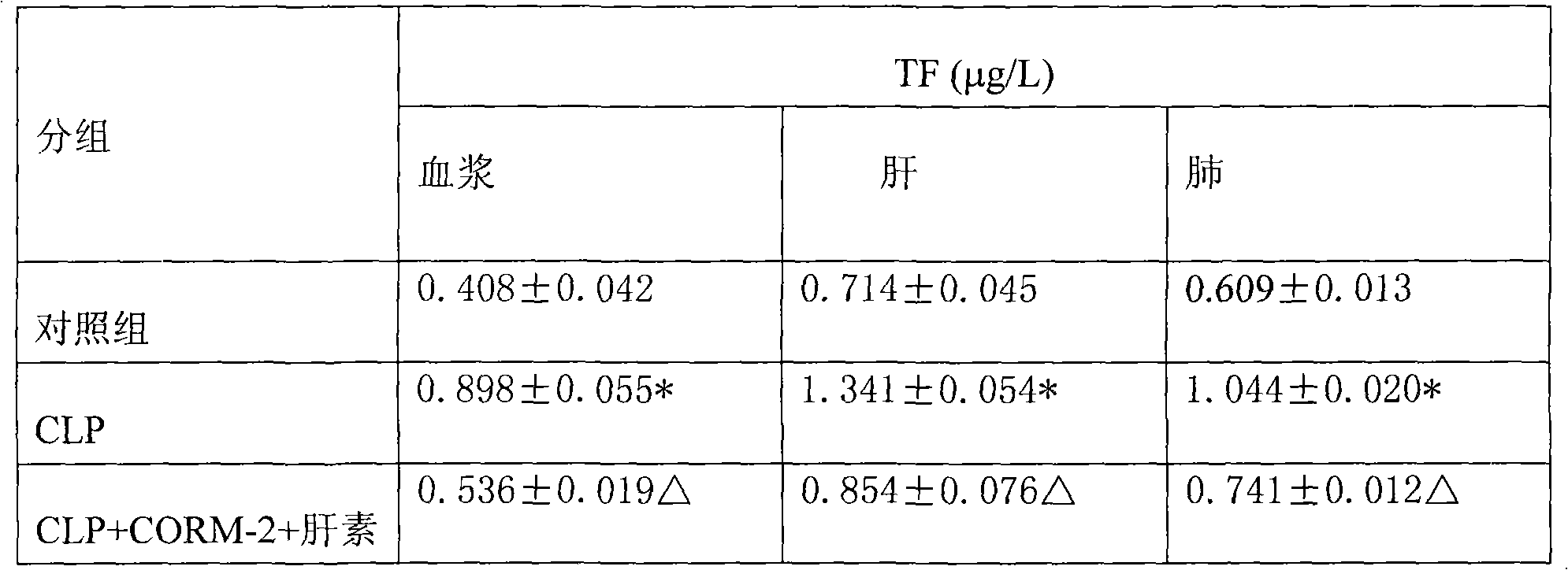
Application of carbon monoxide releasing molecules in preparing medicines for inhibiting blood coagulation activation diseases
InactiveCN102813925AImprove convenienceMeet the needs of clinical applicationInorganic active ingredientsBlood disorderDiseasePhosphorylation
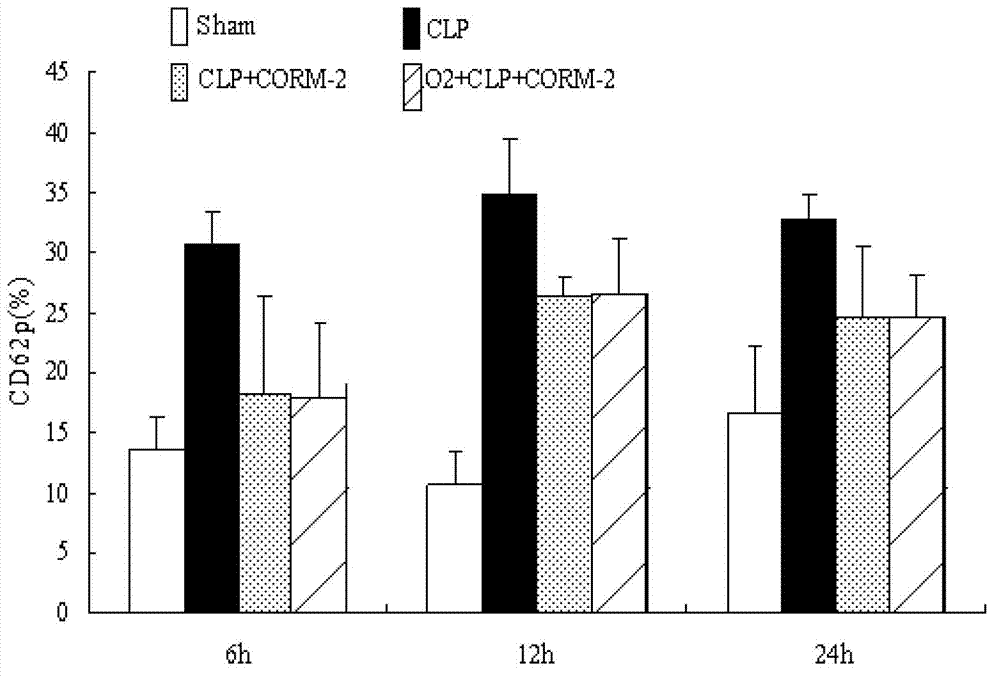
The invention discloses application of carbon monoxide releasing molecules in preparing medicines for inhibiting blood coagulation activation diseases. In vivo animal experiments and in vitro cell experiments show that exogenous carbon monoxide can significantly reduce the expression of blood coagulation factors FIB and D-D in sepsis, and reduce the expression of platelet membrane glycoprotein CD 61 and CD 62p in plasma, thereby down-regulating the adhesiveness and aggregation function of platelet, inhibiting the phosphorylation level of platelet HIS of sepsis mice and finally inhibiting over activation of the coagulation system in sepsis. When the carbon monoxide releasing molecules are adopted for inhibiting over activation diseases of the coagulation system in sepsis, the concentration control is relatively easy and accurate, adding is convenient for long-term use, and the requirement of clinical application can be satisfied.
Owner:AFFILIATED HOSPITAL OF JIANGSU UNIV
Application of carbon monoxide release molecules in preparing medicament for treating early sepsis
InactiveCN101590228AConcentration control is easy and correctImprove convenienceInorganic active ingredientsAntiinfectivesBiological bodyMedicine
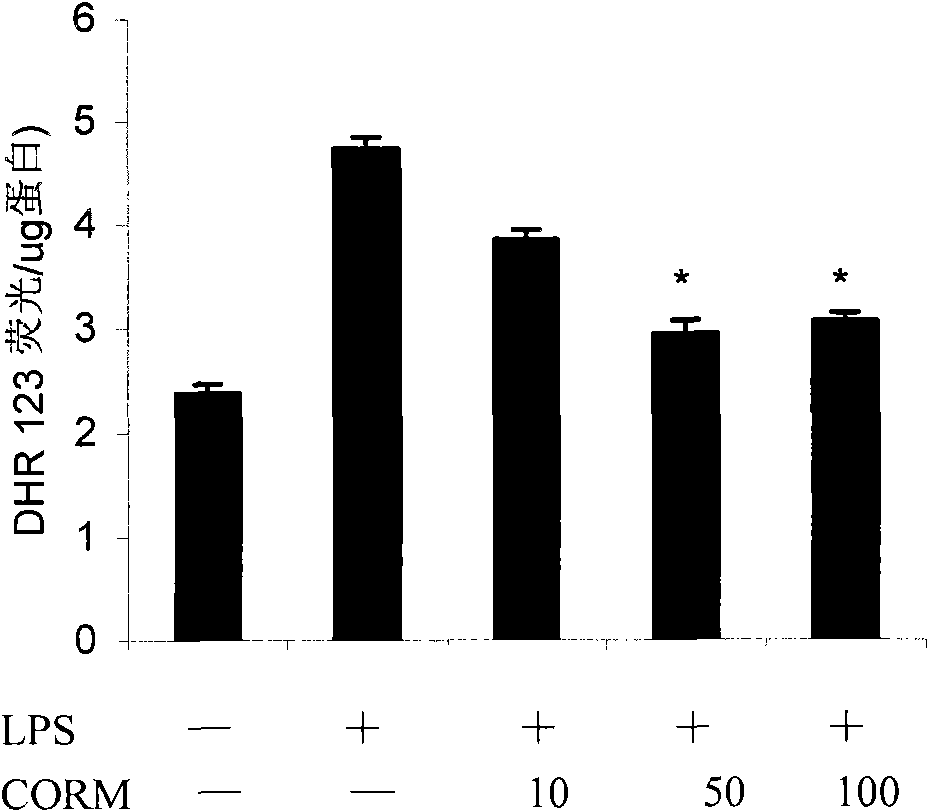
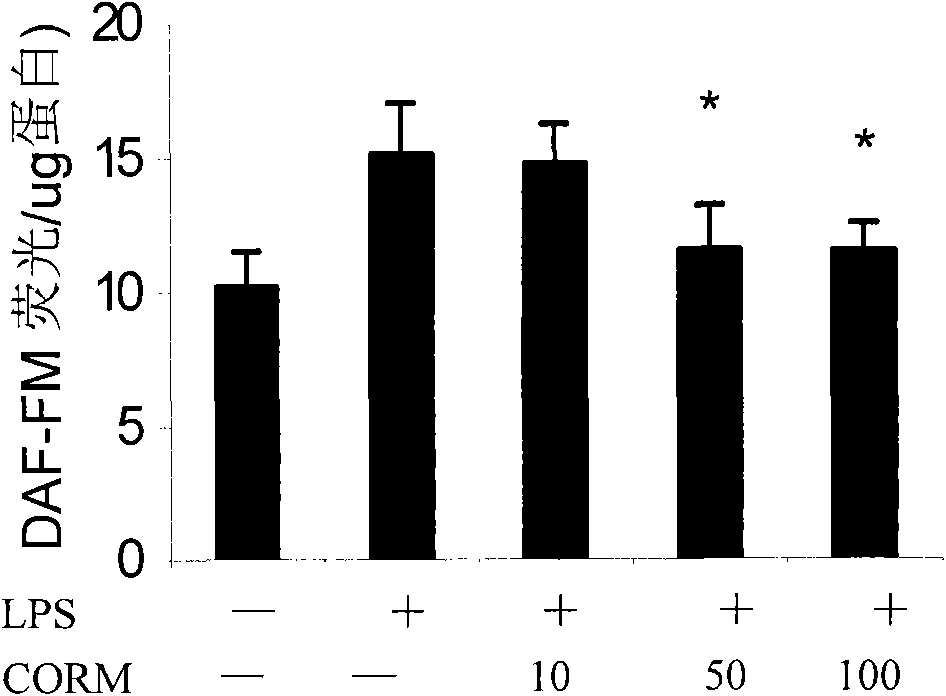
The invention discloses an application of carbon monoxide release molecules in preparing a medicament for treating early sepsis. The invention discovers, according to massive cell and animal tests, that when the carbon monoxide release molecules are dissolved in DMSO solvent and then positioned in cell supernatant or a biological body, CO can be sustainedly released, therefore, the carbon monoxide release molecules can be used as an exogenous CO supplier; in addition, an inflammation model and a mouse CLP sepsis model which are stimulated by cells LPS are adopted for early intervention, and after tests, the carbon monoxide release molecules are dissolved to be capable of serving as a medicament for inhibiting the early sepsis. If the carbon monoxide release molecules are used for inhibiting the sepsis, the control of the concentration thereof is relatively easy and correct, and the superaddition is convenient if the carbon monoxide release molecules are used for a long time. The carbon monoxide release molecules can meet the requirements of clinical application.
Owner:AFFILIATED HOSPITAL OF JIANGSU UNIV
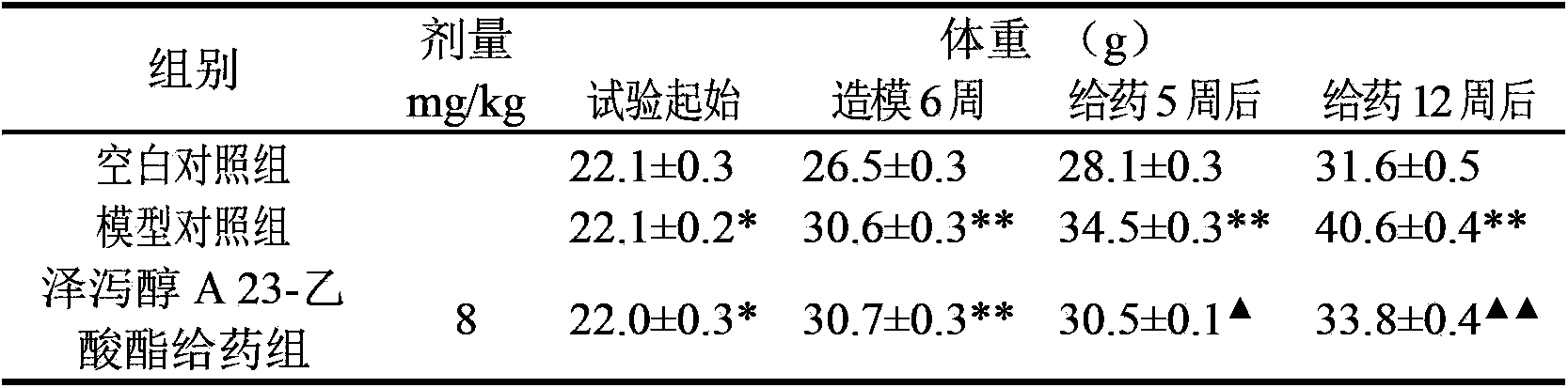
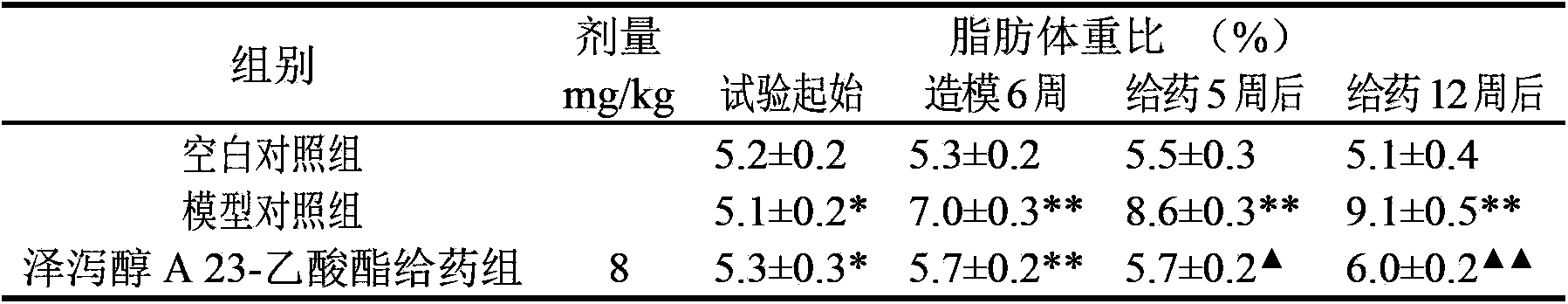
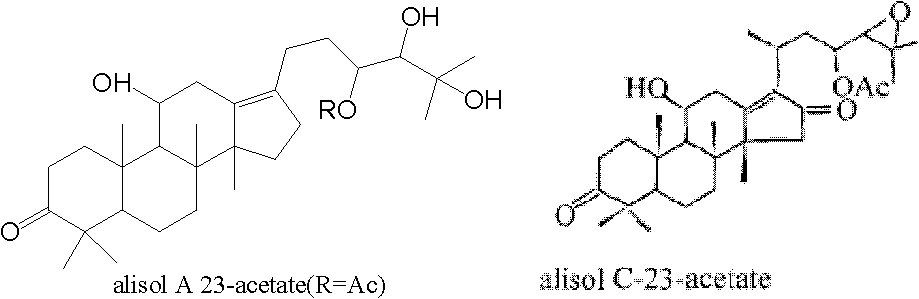
Application of alisol A 23-acetate in preparing drug for treating adiposis
InactiveCN103816162ADefinite curative effectClear ingredientsOrganic active ingredientsMetabolism disorderModern medicineCurative effect
The invention discloses an application of alisol A 23-acetate in preparing a drug for treating adiposis. With the adoption of the application, a modern medicine theory is applied, and the drug for treating the adiposis with definite therapeutic effect, clear compositions and controllable quality is provided, has the obvious actions of weight reduction, fat reduction and adiposity prevention and can satisfy the needs of clinical applications.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Application of carbon monoxide-releasing molecules and heparin in preparing medicament for treating sepsis
InactiveCN101642570AImprove blood coagulation disordersReduce dosageOrganic active ingredientsAntiinfectivesCoagulation defectsSide effect
The invention provides application of carbon monoxide-releasing molecules and heparin in preparing a medicament for treating sepsis. The carbon monoxide-releasing molecules are combined with the ultramicro heparin to improve the coagulation defects of the sepsis, the control of the concentration of the carbon monoxide-releasing molecules and the heparin is relatively easy and correct, the carbon monoxide-releasing molecules and the heparin can be conveniently added when used for a long time, simultaneously the consumption and side effect of the heparin are reduced, and the carbon monoxide-releasing molecules and the heparin can meet the requirement of clinical application. The carbon monoxide-releasing molecules are combined with the ultramicro heparin to improve the coagulation defects ofthe sepsis, the control of the concentration of the carbon monoxide-releasing molecules and the heparin is relatively easy and correct, the carbon monoxide-releasing molecules and the heparin can beconveniently added when used for a long time, and the carbon monoxide-releasing molecules and the heparin can meet the requirement of clinical application.
Owner:AFFILIATED HOSPITAL OF JIANGSU UNIV
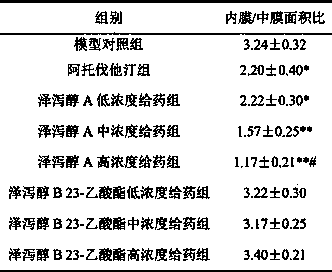
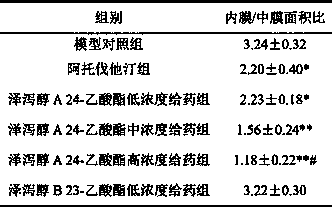
Application of alisol A in preparation of anti-atherosclerosis medicaments
ActiveCN103845339ALow toxicitySuitable for long-term useOrganic active ingredientsCardiovascular disorderIntimal proliferationModern medicine
The invention discloses an application of alisol A in preparation of anti-atherosclerosis medicaments. Animal tests prove that the alisol A has a significant treatment effect on an arteriosclerosis model of an ApoE- / - mouse which is fed with a high-fat diet and combined with carotid artery injury surgery. The medicament disclosed by the invention has relatively low toxicity and is suitable for long-term use. The invention provides the medicament for treating arteriosclerosis, which has the advantages of exact curative effect, definite ingredients and controllable quality, and can meet the needs of clinical applications, by applying the modern medicine theory. The effect of inhibiting vascular intimal hyperplasia of the ApoE- / - mice after carotid artery injuries, caused by high-fat diets, of the alisol A is firstly disclosed by the invention, thereby showing that the alisol A has an obvious effect on treating the arteriosclerosis.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Pharmaceutical preparation containing cyclin inhibitor, and preparation method thereof
InactiveCN105616418AImprove dissolution behaviorImprove bioavailability in vivoOrganic active ingredientsCapsule deliverySulfonatePharmaceutical drug
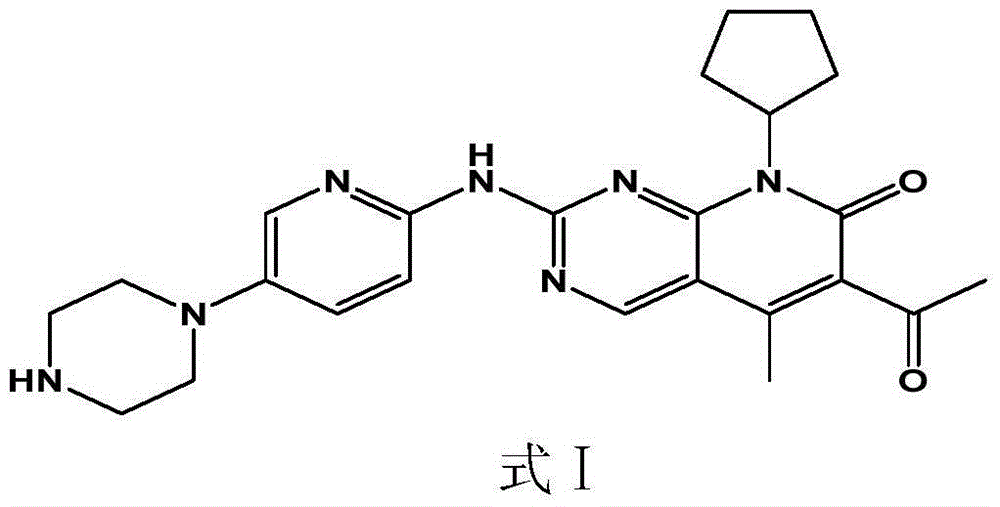
The present invention discloses a pharmaceutical preparation containing a cyclin inhibitor, and a preparation method thereof, and particularly relates to a pharmaceutical preparation adopting 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazine-1-yl-pyridine-2-yl-amino)-8H-pyrido[2,3-d]pyrimidine-7-one represented by a formula I or a salt thereof as an active component, wherein the salt comprises a hydrochloride and an isethionate, and the dosage form of the pharmaceutical preparation comprises tablets and capsules, and has good stability and excellent dissolution behavior. The formula I is defined in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Muscular function appliance for preventing and treating early-stage dental-maxillofacial deformities
The invention discloses a muscular function appliance for preventing and treating early-stage dental-maxillofacial deformities. The basic structure of the muscular function appliance comprises a lip and cheek screen, upper and lower tooth eruption rails, lip thorns, a tongue ring, a tongue bracket, breathing holes, a lip ring hole and a lip ring. The appliance mainly corrects the bad maxillofacial muscular functions through the different designs of the parts to influence development of the maxilla and the mandible, the correcting concept is added into the appliance, and therefore the purpose of correcting the dental-maxillofacial deformities is achieved. According to the appliance, development of the maxilla or the mandible is stimulated by adjusting the height of the lip and cheek screen and the positions of the lip thorns according to the pathogenesis and the disease symptoms of a patient; the surface of the appliance is provided with the breathing holes and the lip ring hole (used by being matched with the lip ring), and the breathing holes and the lip ring hole are used for mouth breathing habit correcting and lip muscle training respectively; the tongue ring is mainly used for guiding a tongue body to be placed at the correct position to coordinate the relative position relation of the maxilla and the mandible. The prepared muscular function appliance is better in pertinence, more stable in treatment effect, more convenient to use and capable of meeting the requirements of orthodontics of the patient and clinical application.
Owner:NANJING MEDICAL UNIV
Human myoblast exosome preparation method, product and application thereof
InactiveCN108795852ASimple manufacturing methodMeet the needs of clinical applicationCosmetic preparationsToilet preparationsCentrifugationExosome
The invention belongs to the technical field of skin cell damage repair, and in particular relates to a human myoblast exosome preparation method, a product and an application thereof. Under the separation condition at the specific temperature, the multi-stage incremental centrifugation mode is adopted to conduct centrifugation for more than 3 times to remove a precipitate till a desired human myoblast exosome product is obtained. The preparation method is simple and feasible. The human myoblast exosome is derived from human myoblasts, contains various biological active substances such as lipids, proteins, mRNA and miRNA, and has obvious effects on daily skin care and damage repair. The myoblast exosome is made into lyophilized powder that is convenient to store and transport and can alsobe made into various skin care products or pharmaceutical preparations to provide the possibility for human skin care and skin damage repair by the human myoblast exosome.
Owner:HUAZHONG UNIV OF SCI & TECH
Application of alisol A 24-acetate in preparing drug for treating adiposis
InactiveCN103816161ADefinite curative effectClear ingredientsOrganic active ingredientsMetabolism disorderAcetic acidModern medicine
The invention discloses an application of alisol A 24-acetate in preparing a drug for treating adiposis. Proved by an animal test that the alisol A 24-acetate has remarkable therapeutic effect to an obesity model caused by feeding with a high-fat feed and can be used for preparing the drug for treating and preventing the adiposis. With the adoption of the application, a modern medicine theory is applied, and the drug for treating the adiposis with definite therapeutic effect, clear compositions and controllable quality is provided and can satisfy the needs of clinical applications. The invention firstly discloses that the alisol A 24-acetate is used for inhibiting weight and fat increase of a mouse caused by a high-fat diet, has the obvious effects of weight reduction, fat reduction and adiposity prevention and has important significance on the clinical applications.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Composition containing alisol A and application of composition containing alisol A on medicine
ActiveCN103054877AGood hypolipidemic effectImprove druggabilityOrganic active ingredientsMetabolism disorderAcetic acidMedicine
The invention discloses a composition containing alisol A and an application of the composition containing alisol A on medicine, and the composition containing alisol A comprises the following ingredients by weight, alisol A: alisol A-23-acetate: alisol A-24-acetate is 1: 0.05-0.3: 0.05-0.5. The composition has the advantages of stable quality and high security, compared with single administration of alisol A or alisol A-23-acetate or alisol A-24-acetate, the composition containing alisol A with long-time administration has better hypolipemic effect by using same dosage with the alisol A or alisol A-23-acetate or alisol A-24-acetate, and in-vitro hepatotoxicity test results display that compared with single administration of alisol A or alisol A-23-acetate or alisol A-24-acetate with same dosage, the composition presents weak performance on cytotoxicity of hepatocyte. The alisol A-23-acetate combines with alisol A or alisol A-24-acetate to obtain the composition, the stability of alisol A-23-acetate is obviously increased, and the druggability is enhanced.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Application of alisol A 24-acetate in preparation of medicaments for preventing and treating arterial diseases
ActiveCN103845340ALow toxicitySuitable for long-term useOrganic active ingredientsCardiovascular disorderModern medicineAdditive ingredient
The invention discloses an application of alisol A 24-acetate in preparation of medicaments for preventing and treating arterial diseases. Animal tests prove that the alisol A 24-acetate has a significant treatment effect against an arteriosclerosis model of an ApoE- / - mouse which is fed with a high-fat diet and combined with carotid artery injury surgery. The medicament disclosed by the invention has relatively low toxicity and is suitable for long-term use. The invention provides the medicament for treating arteriosclerosis, which has the advantages of exact curative effect, definite ingredients and controllable quality, and can meet the needs of clinical applications, by applying the modern medicine theory.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Preparation method of latex particles coated with prostate specific antigen-antibody and PSA enhanced turbidimetric immunophelometry kit
ActiveCN102901810BImprove featuresHigh sensitivityMaterial analysis by observing effect on chemical indicatorPSA AntibodyPsa antigen
The invention relates to a preparation method of latex particles coated with a prostate specific antigen-antibody (PSA) and a PSA-enhanced turbidimetric immunophelometry kit. The latex particles coated with the PSA antibody are prepared by bonding the PSA antibody with latex particles by an optimized physical adsorption method. According to the invention, the kit utilizes the PSA antibody bonded onto the surface of the latex particles and the PSA in the human blood sample to conduct immunoreaction to form the turbidity, and the kit detects the content of the prostate specific antigen-antibody by the increase of the turbidity.
Owner:BEIJING STRONG BIOTECH INC
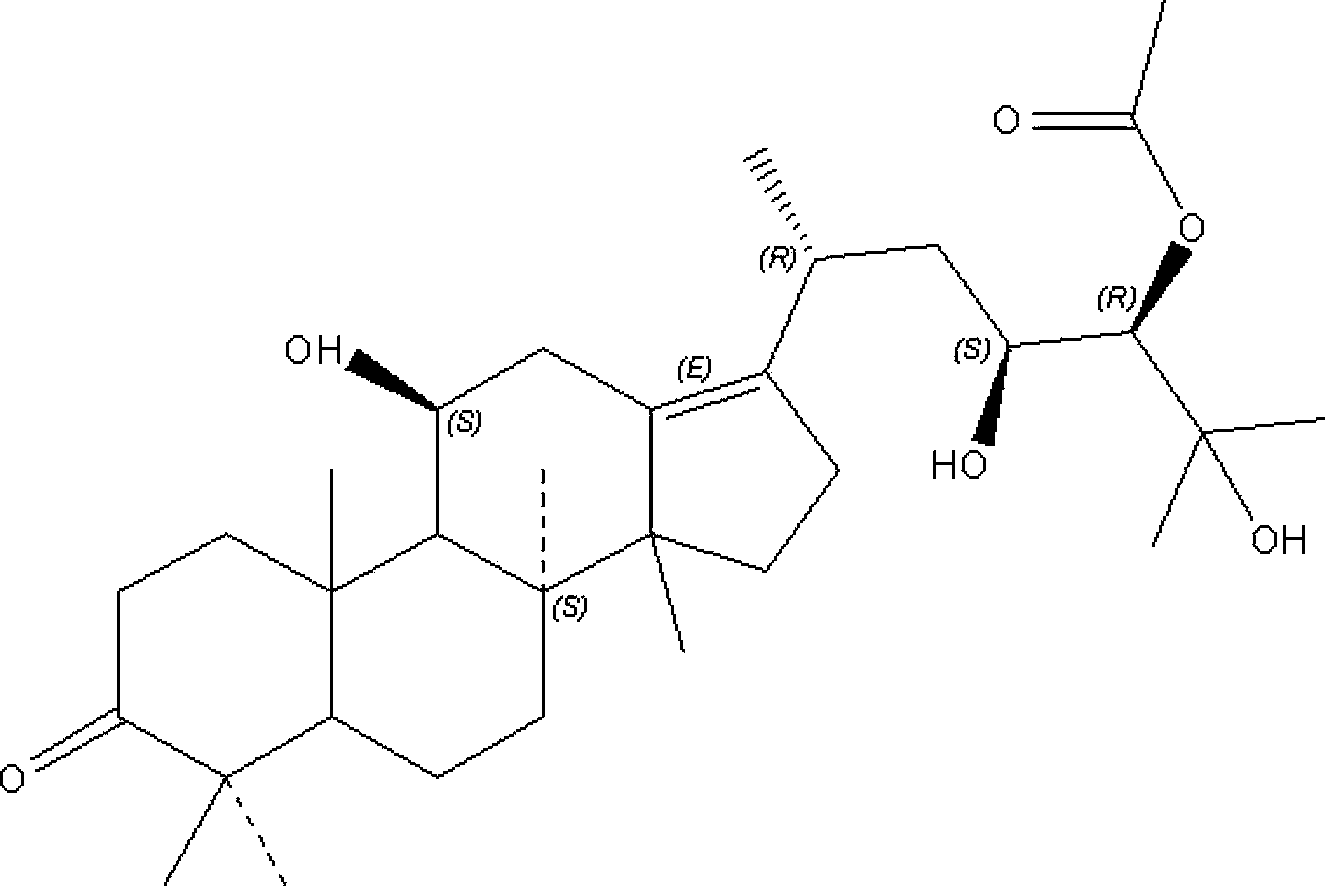
Panaxoside composition as well as preparation method and application thereof
InactiveCN104116746AAvoid toxicity of intolerable high-dose chemotherapy drugsAvoid therapeuticOrganic active ingredientsAntineoplastic agentsEpoxyClinical efficacy
The invention discloses a panaxoside composition as well as a preparation method and application thereof. The panaxoside composition comprises the following components in percentage by weight: 10-80 percent of 20(S)-protopanoxadiol, 1-20 percent of 25-alkene-20(S)-protopanoxadiol, 5-50 percent of 20(S) and 24(R)-panaxatriol epoxy compounds, 5-50 percent of 20(S)-panaxatriol, 1-20 percent of 20(S)-24-methyl-23-alkene-24-carbonyl-protopanoxadiol and 1-20 percent of 25-hydroxy-23-alkene-20(S)-protopanoxadiol. The panaxoside composition disclosed by the invention has the beneficial effects that the panaxoside composition has remarkable treatment effects of directly inhibiting and killing cancers, reversing the multidrug resistance of cancer cells and enhancing the treatment effects of a cancer chemotherapeutic medicine, is definite in clinical effect and capable of preventing the defects that no other treatment methods are available after drug discontinuance and drug-resisting relapse because lots of cancer patients cannot tolerate the toxicity of the chemotherapeutic medicine, and meeting the needs of clinical application.
Owner:祁展楷
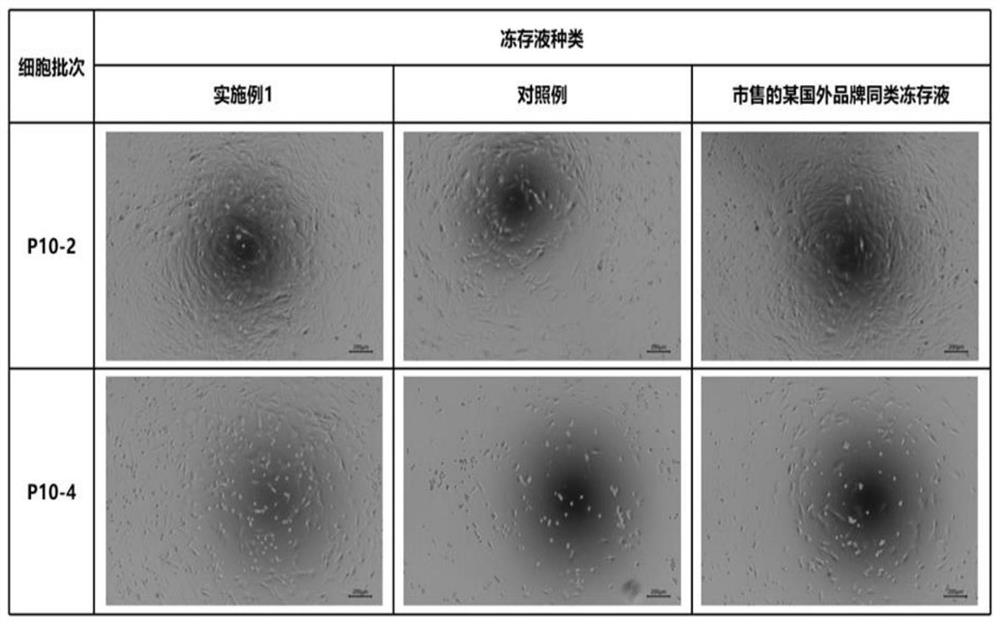
Umbilical cord mesenchymal stem cell protein-free non-programmed freezing medium and preparation method thereof
ActiveCN114557337AAvoid immune responseSmall batch-to-batch varianceDead animal preservationSurface markerInhibitor of apoptosis
The invention relates to the technical field of stem cell culture, in particular to a protein-free non-programmed freezing medium for umbilical cord mesenchymal stem cells as well as a preparation method and application of the protein-free non-programmed freezing medium. The cryopreservation solution comprises a basic solution, a nutritional supplement, a permeable protective agent, a non-permeable protective agent, a cell sedimentation stabilizer, a cell membrane protective agent, an apoptosis inhibitor and an antioxidant, the basic solution is DMEM / F-12 with the glucose content being smaller than or equal to 1000 mg / L. The cryopreservation liquid is suitable for directly cryopreserving umbilical cord mesenchymal stem cells at-80 DEG C after in-vitro amplification and before stem cell treatment. The cryopreservation liquid is free of serum and protein and clear in chemical component, and the cryopreserved umbilical cord mesenchymal stem cells are free of exogenous pollution risk and safer to use; after the cells are recovered, the viability is high, the adherence rate is high, and the cell expansion is fast; surface marker characteristics (phenotypes) and three-line differentiation potential of the mesenchymal stem cells can be maintained; optimization is carried out according to the cryopreservation and culture characteristics of the umbilical cord mesenchymal stem cells, the cryopreservation effect is improved, programmed cooling is not needed, and time and labor are saved.
Owner:大连博格林生物科技有限公司
Non-genetically modified light control bidirectional regulation method for human umbilical cord mesenchymal stem cell proliferation
ActiveCN108624554AObviously positiveThe effect of obvious negative regulationSkeletal/connective tissue cellsElectrical/wave energy microorganism treatmentUmbilical cordMedical treatment
The invention relates to a non-genetically modified light control bidirectional regulation method for human umbilical cord mesenchymal stem cell proliferation. The method includes the steps: irradiating human umbilical cord mesenchymal stem cells at 95-105mm positions under a collimating mirror for 85-95min through the collimating mirror by the aid of blue light under the conditions of the light wavelength of 400-480nm and the light intensity of 95-105 micro-w / cm<2>, and performing negative regulation; or irradiating the human umbilical cord mesenchymal stem cells at the 95-105mm positions under the collimating mirror for 115-125min through the collimating mirror by the aid of blue light, and performing positive regulation. According to the method, the multiplication speed of the human umbilical cord mesenchymal stem cells is firstly and bi-directionally regulated only through blue light irradiation under setting of special parameters, foreign genes transferred into the stem cells areomitted, virus transfection vectors are omitted, any other substances are omitted, and the method more meets clinical application requirements of 'noninvasive light therapy' and has a wide clinical application prospect.
Owner:SHANDONG UNIV
Compound preparation having anti-tumor action
InactiveCN102429914AStrong penetrating powerPromote absorptionOrganic active ingredientsAntineoplastic agentsCelluloseDexamethasone
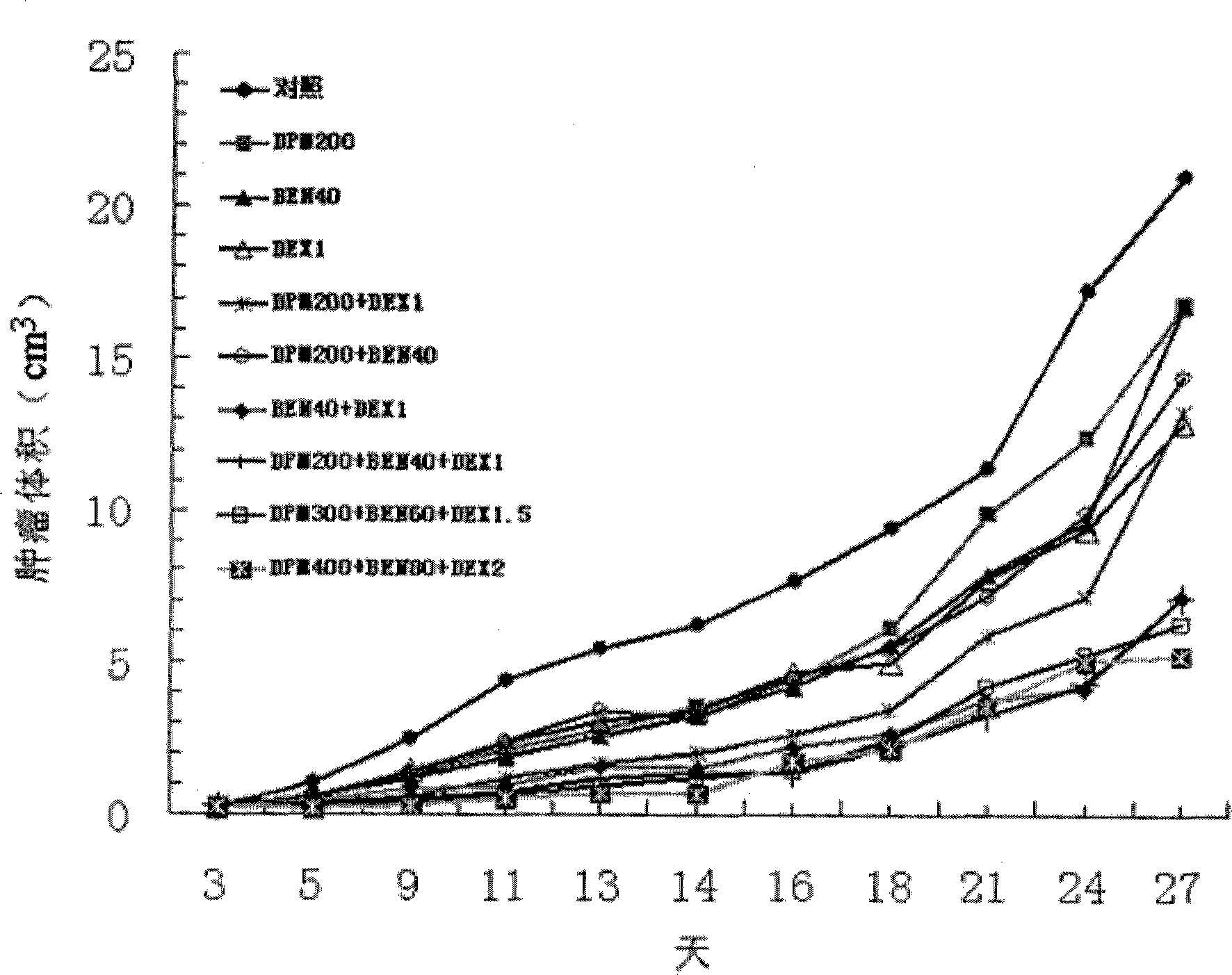
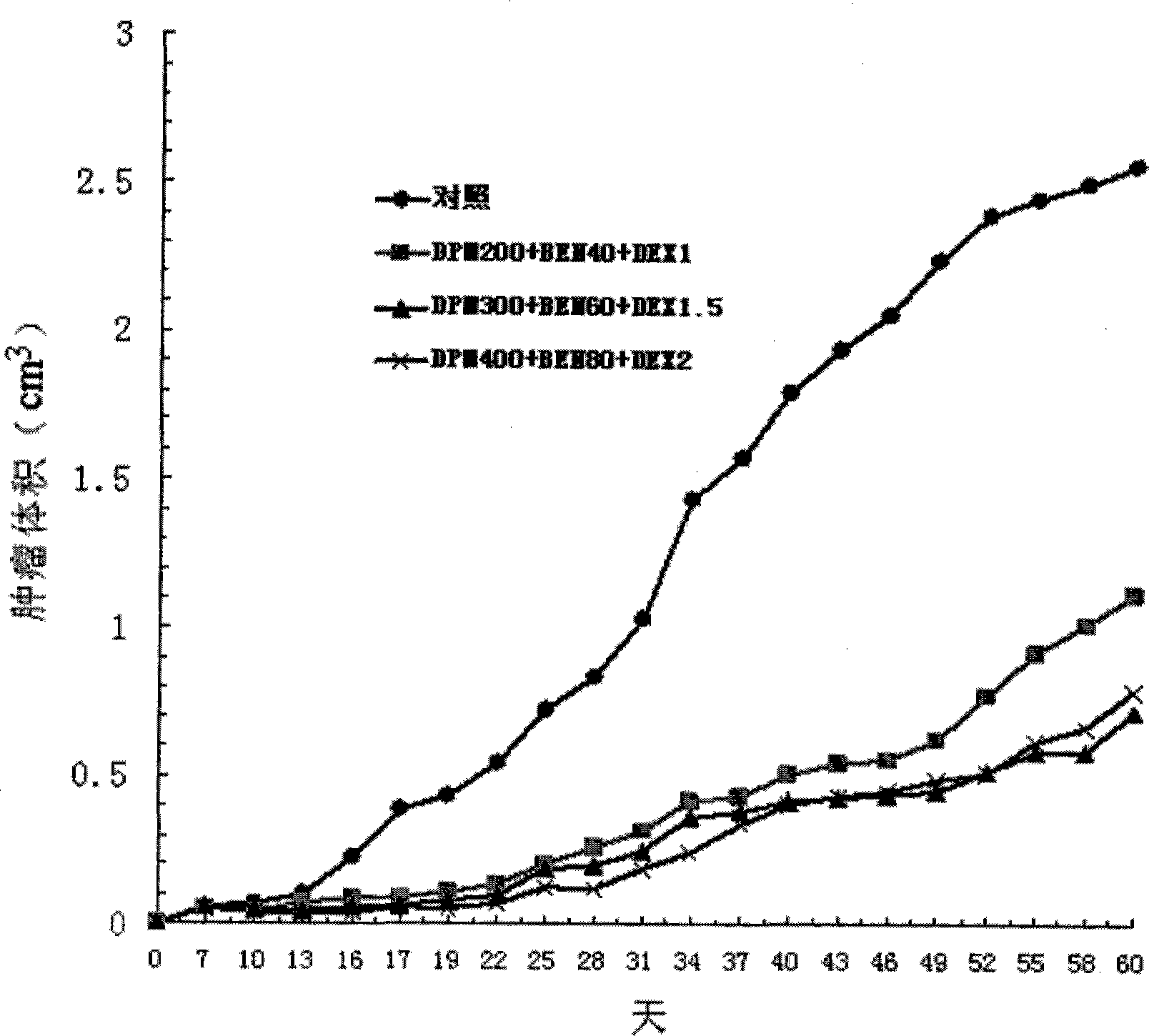
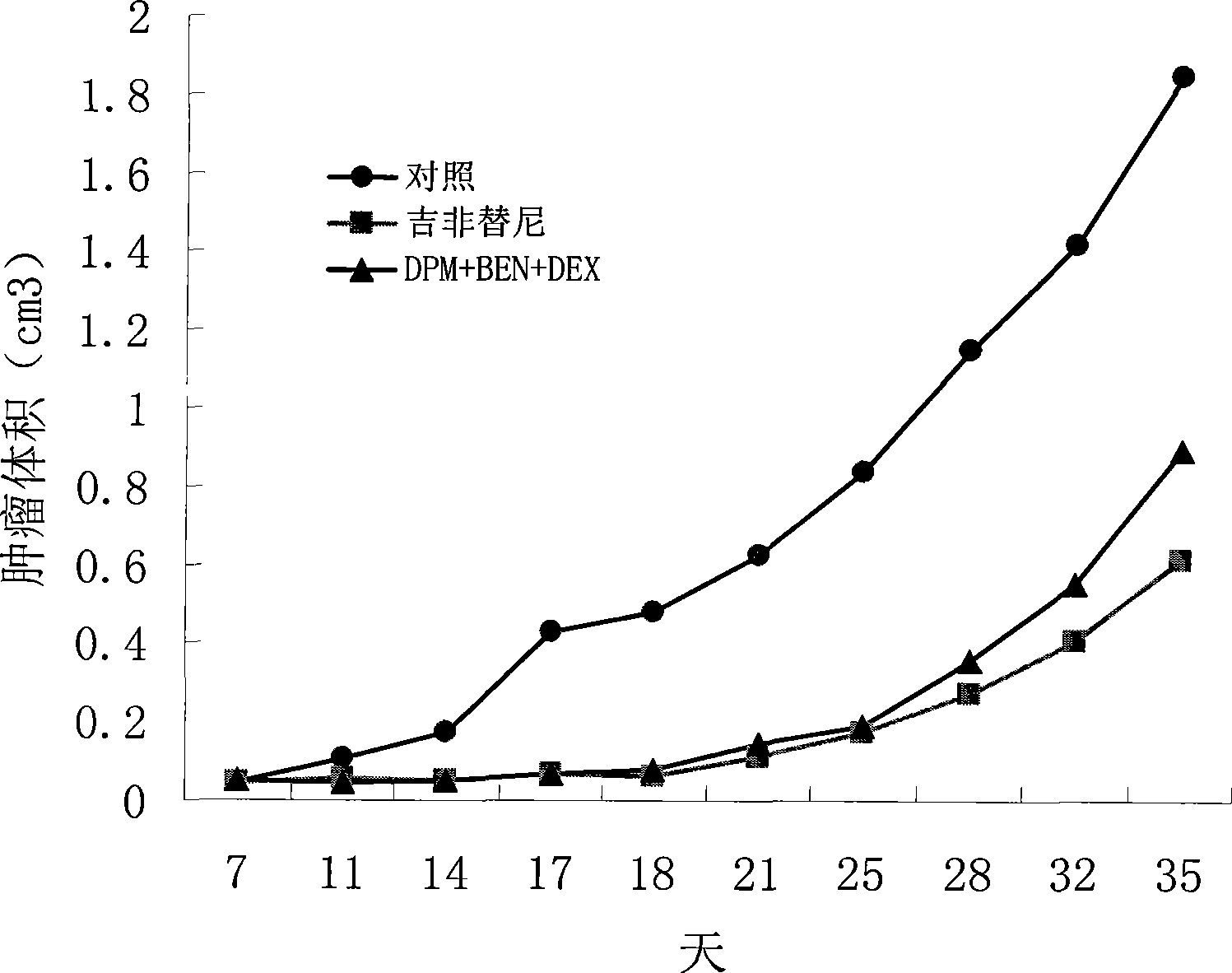
The invention provides a compound preparation having an anti-tumor action. The compound preparation comprises therapeutically effective amounts of ingredient A, ingredient B, ingredient C and a pharmaceutically acceptable carrier; or mixture of the ingredient A, the ingredient B and the ingredient C; or a capsule taking the mixture of the ingredient B and the ingredient C as restricted substances; the ingredient A is enteric coating particles of dipyridamole and is formed by dipyridamole enteric particles and coating layers for packing the dipyridamole enteric particles; the ingredient B is ubenimex adhesion particles taking insoluble cellulose and biological adhesive materials as carriers; and the ingredient C is dexamethasone with a particle size of 100-200 nm. The compound preparation having the anti-tumor action formed by the three compositions can reach the peak value in blood at a higher speed, and can remain longer effective concentration time respectively, so that the requirement of clinical application can be met.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Medicinal composition for treating spondyle disease and method for preparing the same
InactiveCN101095783ASignificant effectNo toxicitySkeletal disorderPlant ingredientsDiseaseSpinal disease
The invention relates to a pharmaceutical composition for treating spinal diseases and process for preparation, wherein the pharmaceutical composition is prepared from the following raw materials (by weight portions): processed himalayan teasel root 8-16 parts, processed eucommia bark 8-16 parts, homalomena rhizome 5-8 parts, drynaria 8-16 parts, Loranthus mulberry mistletoe 8-16 parts, dragon's blood resin 4-16 parts, red sage root 4-8 parts, spatholobus stem 4-10 parts, walnut kernel 8-20 parts, astragalus root 8-20 parts, wolfberry fruit 10-30 parts, futokadsura stem 4-12 parts, Rosa taiwanensis Naka.4-12 parts.
Owner:唐志杰
Articular cartilage restoration and regeneration stent and preparation method thereof
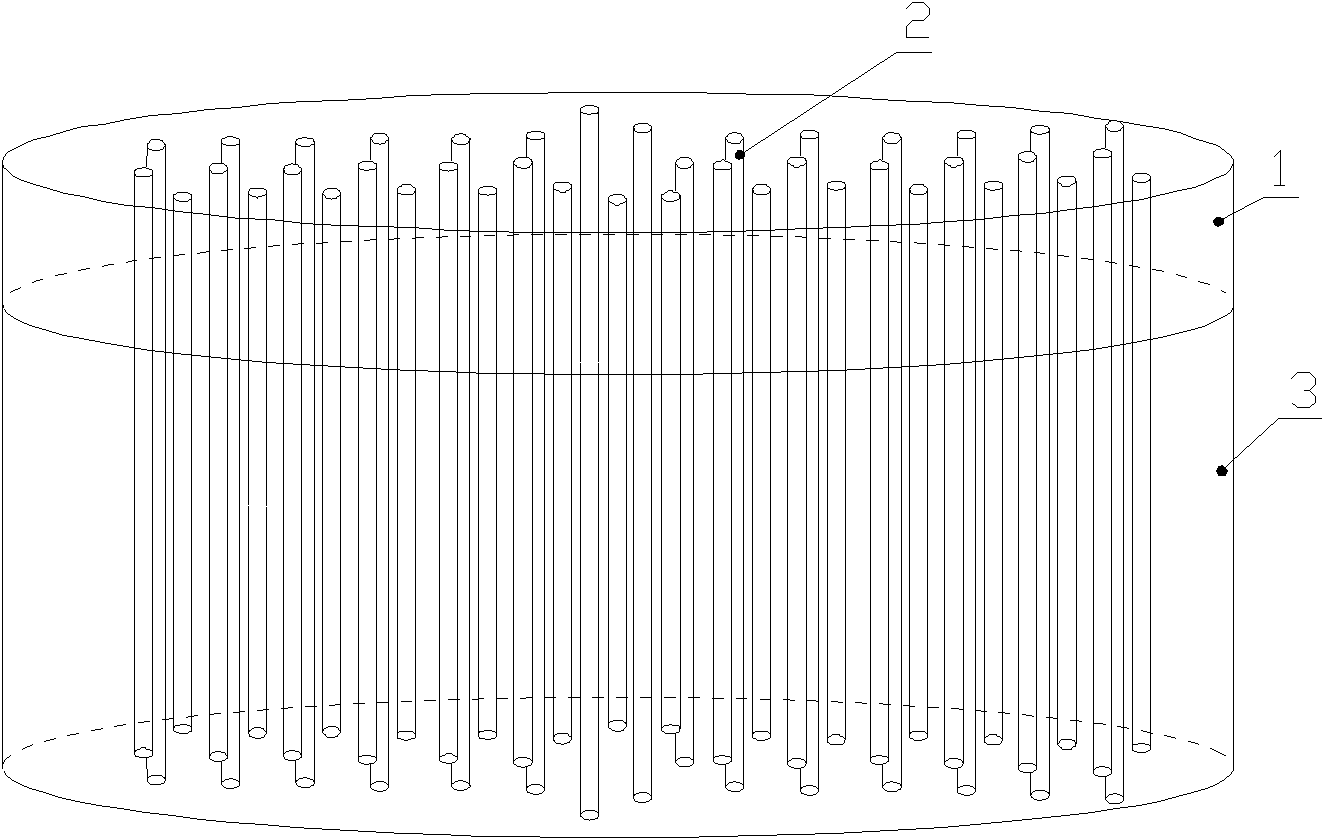
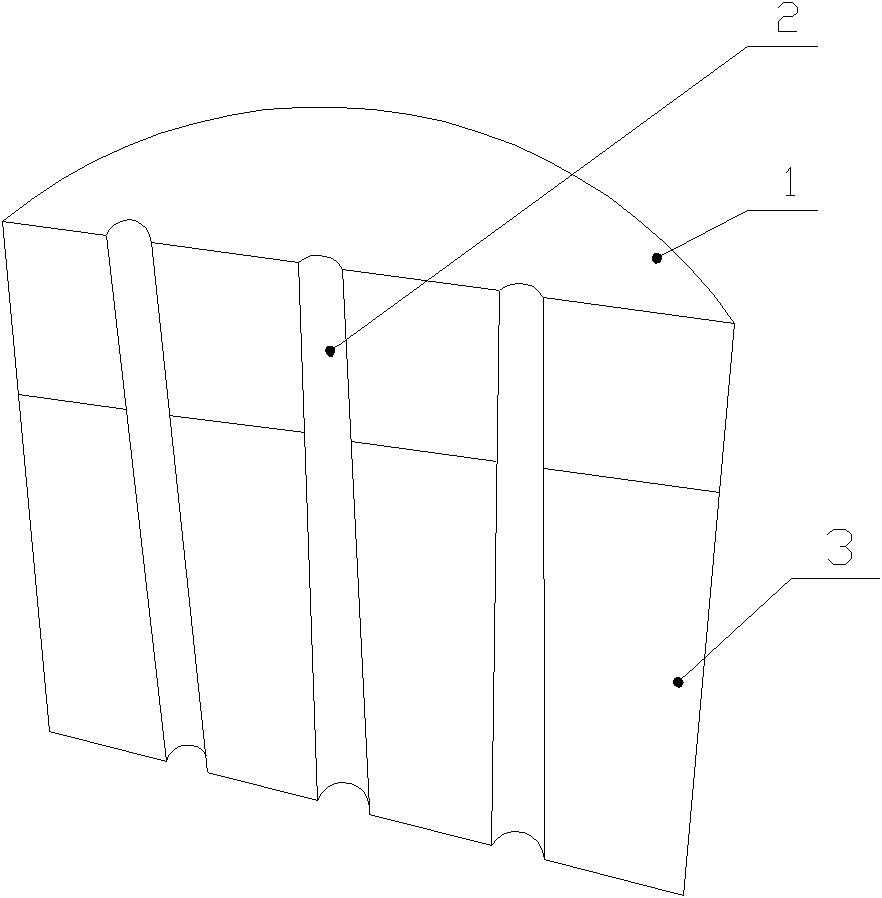
ActiveCN102078642BSufficient and safe sources of donorsEase of clinical popularizationProsthesisBiocompatibility TestingBone Cortex
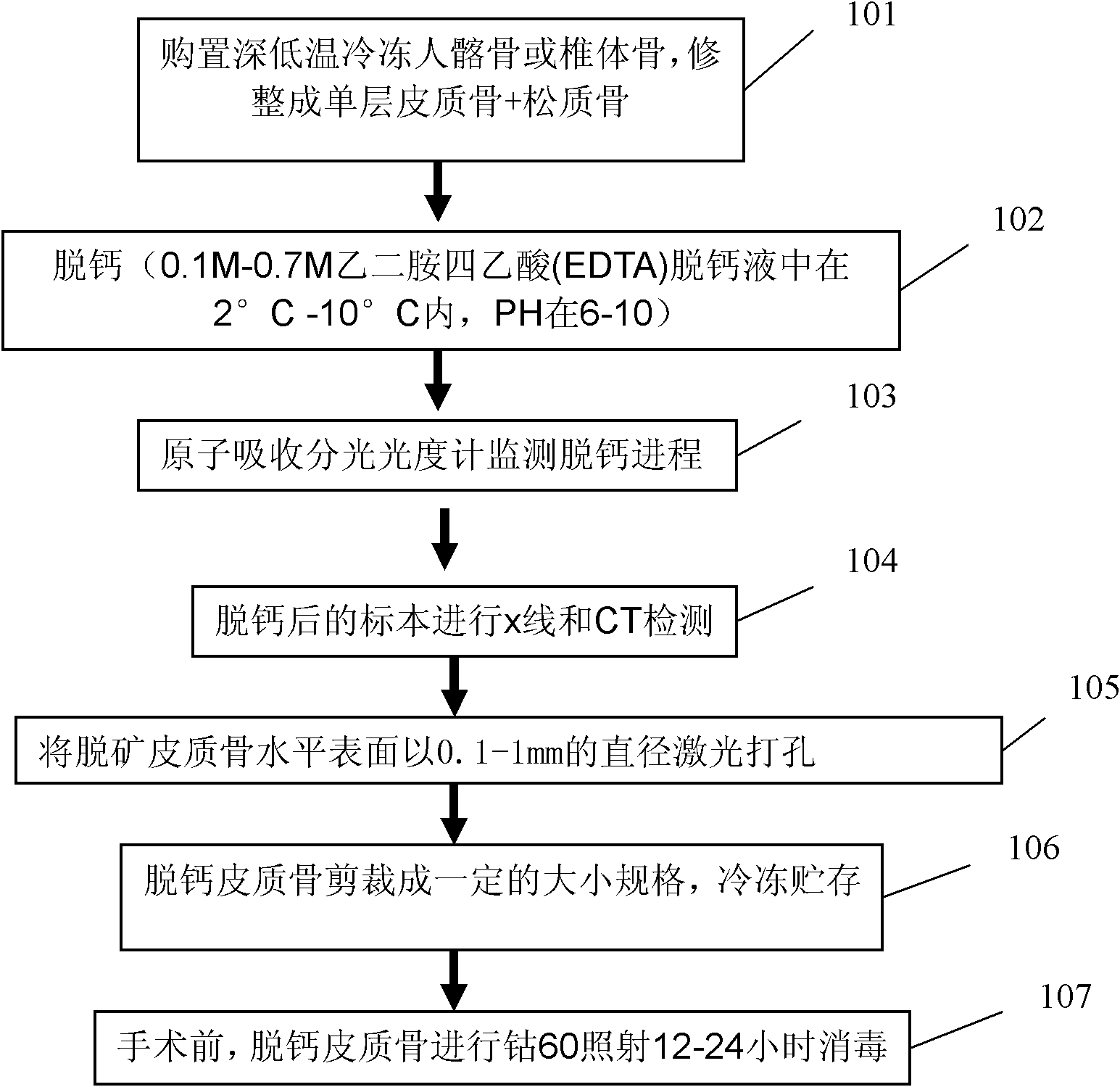
The invention discloses an articular cartilage restoration and regeneration stent and a preparation method thereof, belonging to the field of medical materials. The stent is made of decalcified spongiosa-cortical bone, and the decalcified spongiosa-cortical bone is provided with more than two holes penetrating through the decalcified spongiosa bone and the cortical bone. The holes are vertically arranged on the horizontal surface of the decalcified cortical bone and have diameter of 0.1-1mm. The width between hole centers is 0.5-5mm. The preparation method provided by the invention comprises the steps of: soaking the bone in a decalcified solution for decalcification; monitoring the decalcification process by using an atomic absorption spectrophotometer; carrying out X-ray and CT detection on a decalcified sample to prove that the bone is completely decalcified to obtain the decalcified spongiosa-cortical bone; drilling on the horizontal surface of the decalcified cortical bone by using laser in a diameter of 0.1-1mm; and cutting the decalcified cortical bone into a certain size, and freezing and storing. The articular cartilage restoration and regeneration stent is made of a natural collagen stent material without immunological rejection reaction, is easy to adsorb by cells, and has good biocompatibility and better mechanical strength.
Owner:BEIJING WANJIE MEDICAL DEVICE CO LTD
Method for preparing medical porous alumina based ceramic composite
InactiveCN103073323AImprove mechanical propertiesFacilitate depositionCeramicwareNitrogen atmosphereNitrogen gas
The invention belongs to the field of biological ceramic materials, and discloses a method for preparing a medical porous alumina based ceramic composite. The method comprises the steps that the following materials by mass percent: 1-8% of calcium fluoride, 90-98% of alumina and 1-5% of diopside are prepared; calcium fluoride, alumina and the diopside are mixed and subjected to ball milling to form a mixed powder body; and then the mixed powder body is placed in a graphite jig for compression molding, subjected to hot pressed sintering in an argon or nitrogen atmosphere on the conditions that a sintering temperature is 1400 DEG C, a pressing temperature is 1320 DEG C, and sintering pressure is 30MPa, and subjected to temperature maintaining and pressure maintaining at 1400 DEG C for 30min. The composite prepared by the method has good mechanical property and bioactivity, and the surface of the composite is distributed with micropores, so that growth of a bone tissue is better facilitated. In addition, the method is low in production cost, and has better application prospects in repairing and replacing a bone of a human body.
Owner:SHANDONG UNIV
Sublimating and refining method for guilingji capsules preparation
InactiveCN108339079ALarge outputImprove efficiencyPowder deliveryNervous disorderTemperature controlMedicine
The invention relates to a sublimating and refining method for traditional Chinese medicine compound prescription and particularly relates to a sublimating and refining method for a guilingji capsulespreparation. The object of the invention is to overcome the defects that a conventional technology has difficulty of controlling fire degree, is influenced by weather, may damage environment, needs long time and high labor intensity, and has difficulty of expanded production. The invention provides a sublimating and refining method for guilingji capsules, which can guarantee active components, iseasy to control in quality and is easy to promote, and prepared product guilingji capsules medicine powder thereof. The sublimating and refining method for guilingji capsules includes the following steps: (1) taking medicine powder into an alloy tank and sealing the alloy tank; (2) putting the alloy tank into a slightly larger stainless steel tank, filling the gap between the alloy tank and the stainless steel tank with coarse sand, putting a cover over the stainless steel tank and sealing the stainless steel tank with mud; (3) placing the stainless steel tank in a baking oven and heating upthe stainless steel tank, and keeping the temperature of 70+ / -1 DEG C by using a temperature control system of the baking oven, and sublimating and refining continuously the medicine powder for 17 days; and (4) taking out the medicine powder to obtain the prepared product guilingji capsules medicine powder after lowering the temperature.
Owner:山西广誉远国药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com