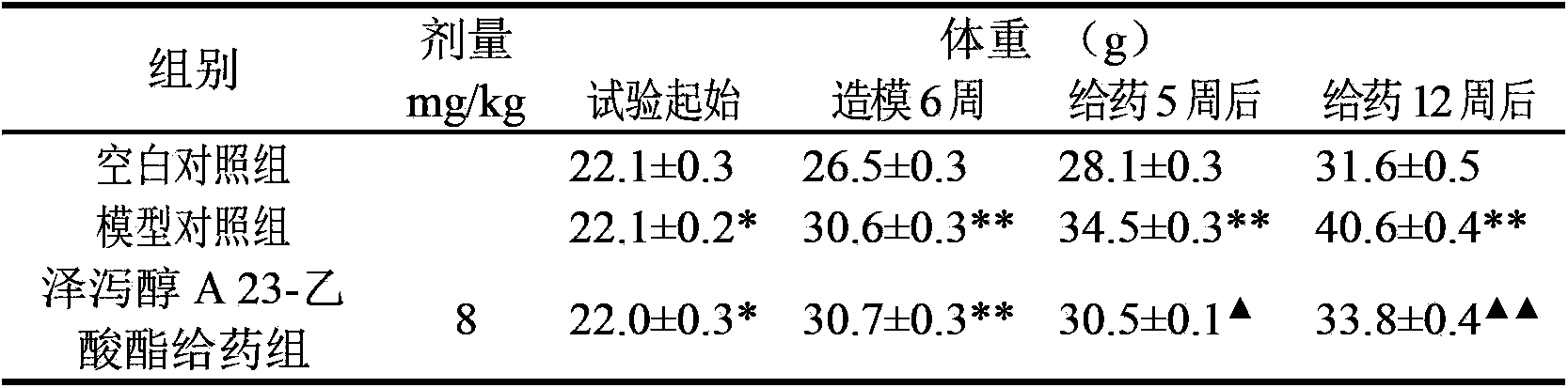
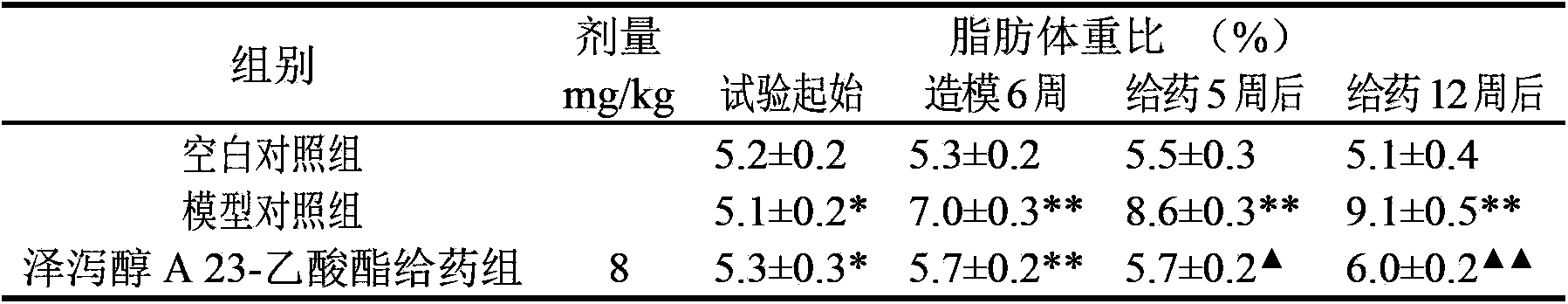
Application of alisol A 23-acetate in preparing drug for treating adiposis
A technology of acetate and alisitol, which is applied in the application field of alisitol A 23-acetate in the preparation of drugs for treating obesity, can solve the problem of weak in vitro inhibition of tumor cell proliferation, unclear effective material basis, The effect of weight loss is not obvious, etc., to meet the clinical application, the ingredients are clear, and the effect is definite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Extraction of medicinal materials:
[0023] Method 1: Take 1 kg of dried tubers of Alisma orientalis (Sam.) Juzep., a plant of the family Alismaceae, make decoction pieces, and reflux extract with 7500ml of ethyl acetate twice, each time for 0.5 hours. The extracts were combined, and the solvent was recovered under reduced pressure to obtain 40 g of dark brown extract.
[0024] Method 2: Take 3 kilograms of dry tubers of Alisma orientalis (Sam.) Juzep., crushed into coarse particles, leak with 75% ethanol, combine the seepage, recover the solvent under reduced pressure, and obtain dark brown infusion Cream 110g.
[0025] Method 3: Take 2 kg of dry tubers of Alisma orientalis (Sam.) Juzep., crush them into coarse particles, and place them in CO 2 In supercritical extractor, extraction condition: CO 2 The pressure is 24-35MPa, the extraction temperature is 41-55°C, the extraction time is 1.5h, and 65g of dark brown extract is obtained.
[0026] Method 4: Take 1 kg of ...
Embodiment 2
[0028] Preparation of Alisitol A 23-acetate:
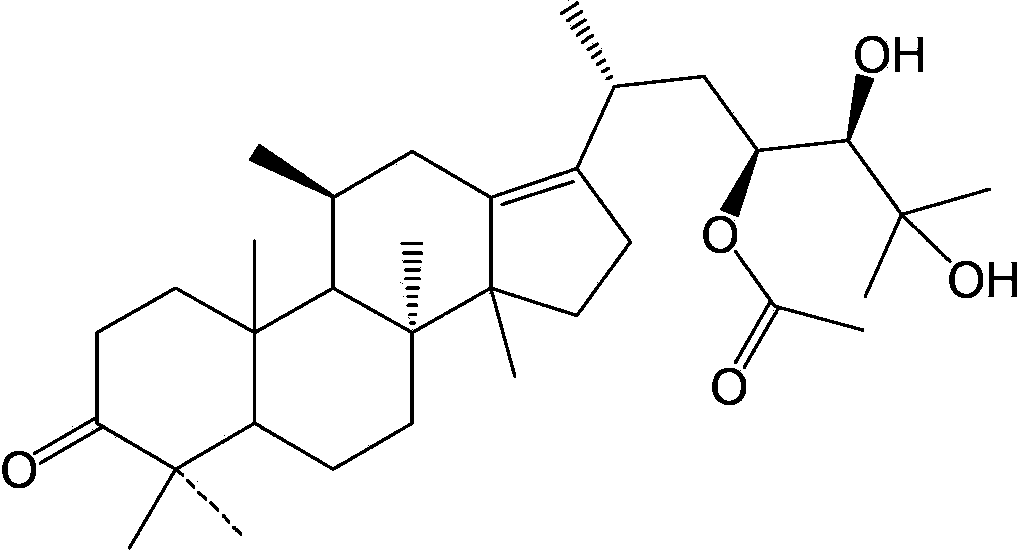
[0029] Take by weighing 10 g of the extract of Example 1, put it on D101 type macroporous adsorption resin, after eluting 10 column volumes with 20% ethanol, use 70% ethanol to elute 5 column volumes instead, collect 70% ethanol eluate, reduce The solvent was recovered under pressure to obtain 2 g of yellow oil; 2 g of yellow oil was dissolved in 65% methanol, and the target compound was separated by preparative HPLC. The separation conditions are: C18 column, mobile phase is 65% methanol, flow rate is 50ml / min, detection wavelength is 208nm. This gave 100 mg of alisol A 23-acetate with a purity of 95% by weight.
[0030] High resolution mass spectrum: m / z: 555.36561, C 32 h 52 o 6 Na + [M+Na], its molecular formula is C 32 h 52 o 6 .
[0031] C 13 NMR(δ): 223.78, 172.87, 139.56, 136.20, 79.80, 73.62, 72.95, 70.76, 58.442, 49.78, 49.49, 48.81, 42.10, 39.51, 38.33, 35.44, 35.27, 356.78, 34.98, 30.10, 30.10, 3 , 28.12, 26...
Embodiment 3
[0033] Weigh 10 g of alisitol A 23-acetate in Example 2, add 8 g of starch, 2 g of microcrystalline cellulose, and 0.2 g of magnesium stearate, mix well, and adopt methods known in the art to make tablets and capsules doses or granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com