Clinical nutrient formula special for tumor and preparation method of clinical nutrient formula
A nutritional formula, special technology, applied in the application, oil-containing food ingredients, oligosaccharide-containing food ingredients and other directions, can solve the problems of insignificant protein nutritional support, incomplete nutritional components, etc., to reduce pathological platelet aggregation, Immunity-boosting, curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
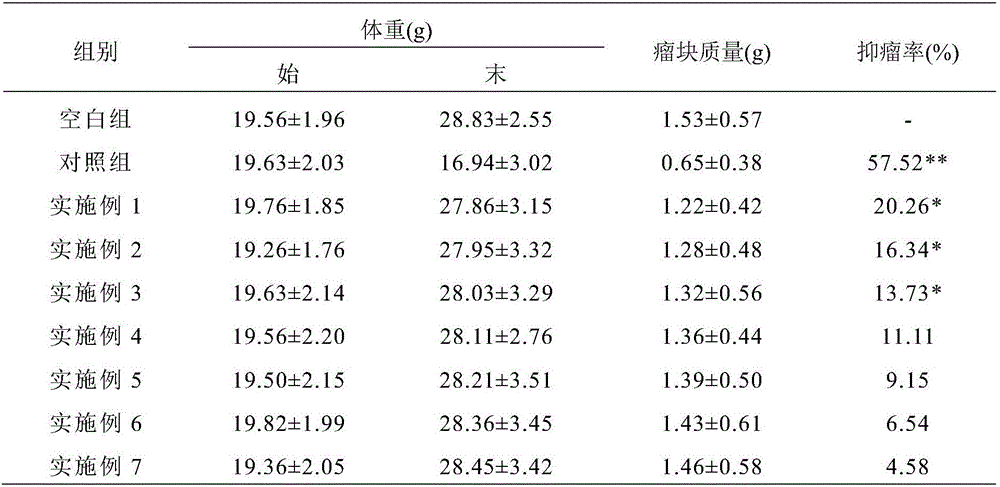
[0055] The tumor-specific clinical nutritional formula of the present invention includes 9.8g of whey protein powder, 5.18g of soybean protein isolate, 26.31g of whole milk powder, 0.18g of whole egg powder, 0.1g of bovine colostrum, 0.11g of taurine, soybean Oligopeptide 0.1g, marine fish oligopeptide powder 0.1g; olive oil 16.6g, soybean oil 4.9g, coconut oil 0.49g, perilla oil 3.49g, medium chain triglyceride 0.49g; maltodextrin 8.25g, Tapioca starch 1.82g, resistant starch 1.82g; dietary fiber selected from inulin, konjac flour, galacto-oligosaccharides, fructo-oligosaccharides, isomalto-oligosaccharides, soybean polysaccharides, cyclodextrin, resistant dextrin, soybean fiber At least one of them, the total mass of which is 7.82g, including 6g of water-soluble dietary fiber and 1.82g of insoluble dietary fiber; 0.4g of calcium, 0.25g of phosphorus, 0.08g of potassium, 0.02g of sodium, and 0.15g of magnesium in macroelements; Iron 0.011g, iodine 0.00014g, zinc 0.007g; fat-s...
Embodiment 2
[0057] The tumor-specific clinical nutritional formula of the present invention includes 7.8g of whey protein powder, 7.18g of soybean protein isolate, 26.31g of whole milk powder, 0.18g of whole egg powder, 0.1g of bovine colostrum, 0.11g of taurine, soybean Oligopeptide 0.1g, marine fish oligopeptide powder 0.1g; olive oil 19.6g, soybean oil 4.9g, coconut oil 0.49g, perilla oil 0.49g, medium chain triglyceride 0.49g; maltodextrin 8.25g, Tapioca starch 1.82g, resistant starch 1.82g; dietary fiber selected from inulin, konjac flour, galacto-oligosaccharides, fructo-oligosaccharides, isomalto-oligosaccharides, soybean polysaccharides, cyclodextrin, resistant dextrin, soybean fiber At least one of them, the total mass of which is 7.82g, including 6g of water-soluble dietary fiber and 1.82g of insoluble dietary fiber; 0.4g of calcium, 0.25g of phosphorus, 0.08g of potassium, 0.02g of sodium, and 0.15g of magnesium in macroelements; Iron 0.011g, iodine 0.00014g, zinc 0.007g; fat-s...
Embodiment 3
[0059]The tumor-specific clinical nutritional formula of the present invention includes 7.8g of whey protein powder, 7.18g of soybean protein isolate, 26.31g of whole milk powder, 0.18g of whole egg powder, 0.1g of bovine colostrum, 0.11g of taurine, soybean Oligopeptide 0.1g, marine fish oligopeptide powder 0.1g; olive oil 19.6g, soybean oil 4.9g, coconut oil 0.49g, perilla oil 0.49g, medium chain triglyceride 0.49g; maltodextrin 11.25g, Tapioca starch 1.82g, resistant starch 1.82g; dietary fiber selected from inulin, konjac flour, galacto-oligosaccharides, fructo-oligosaccharides, isomalto-oligosaccharides, soybean polysaccharides, cyclodextrin, resistant dextrin, soybean fiber At least one of them, the total mass of which is 7.82g, including 6g of water-soluble dietary fiber and 1.82g of insoluble dietary fiber; 0.4g of calcium, 0.25g of phosphorus, 0.08g of potassium, 0.02g of sodium, and 0.15g of magnesium in macroelements; Iron 0.011g, iodine 0.00014g, zinc 0.007g; fat-s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com