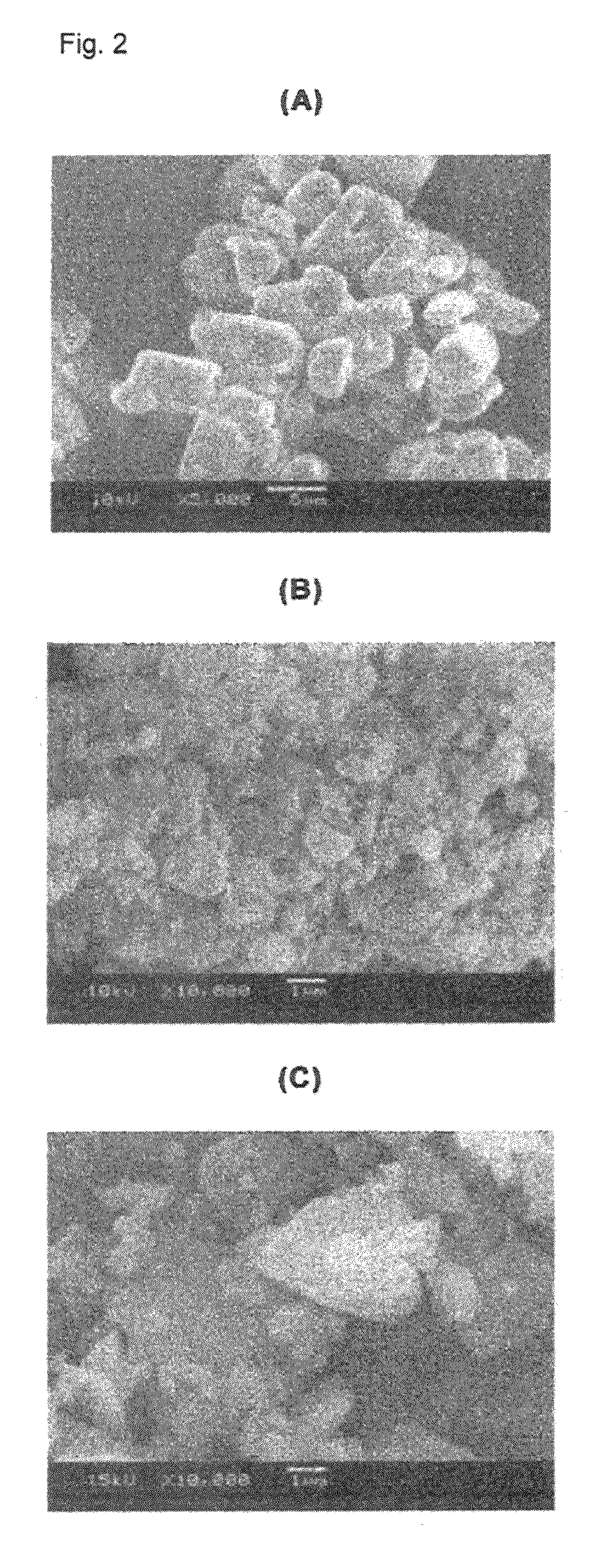
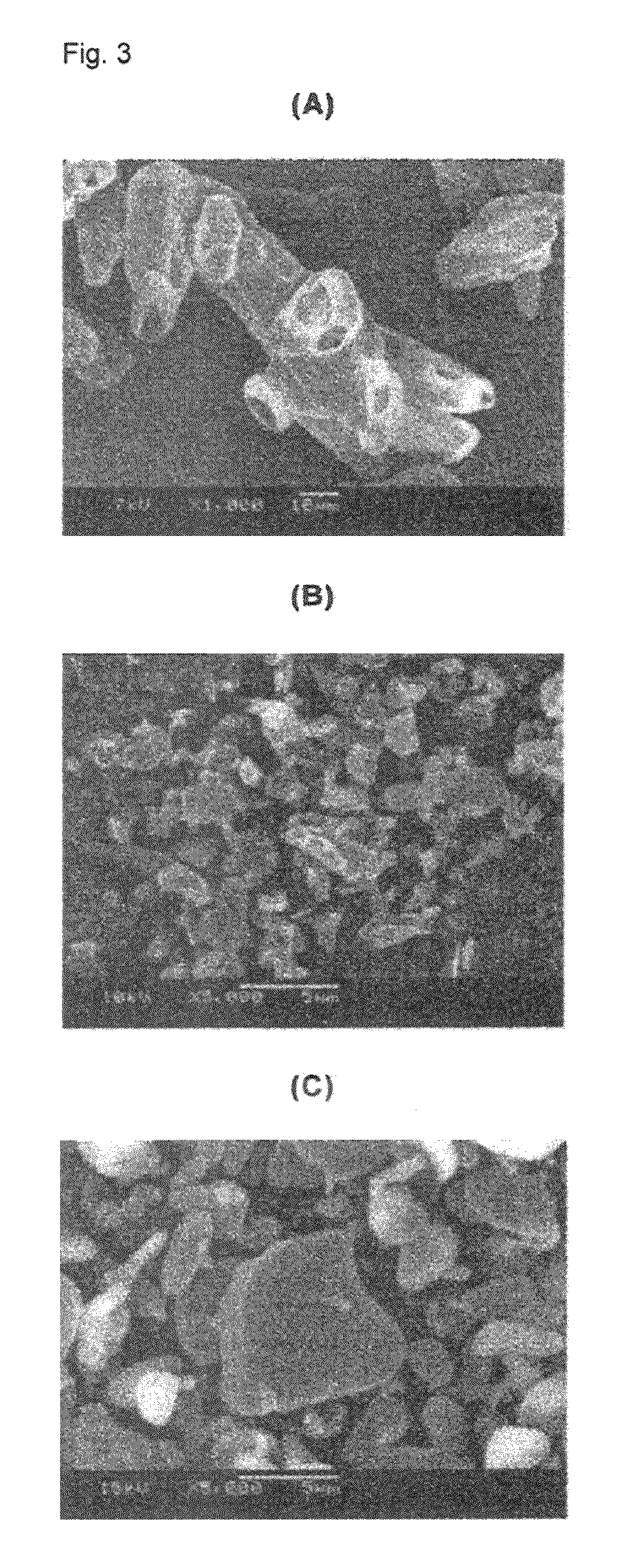
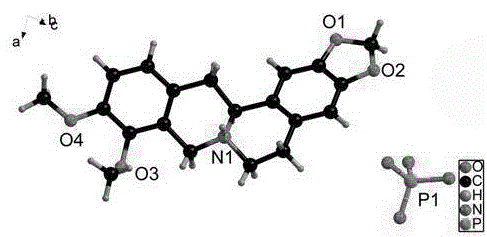
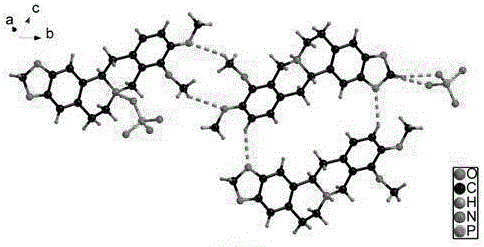
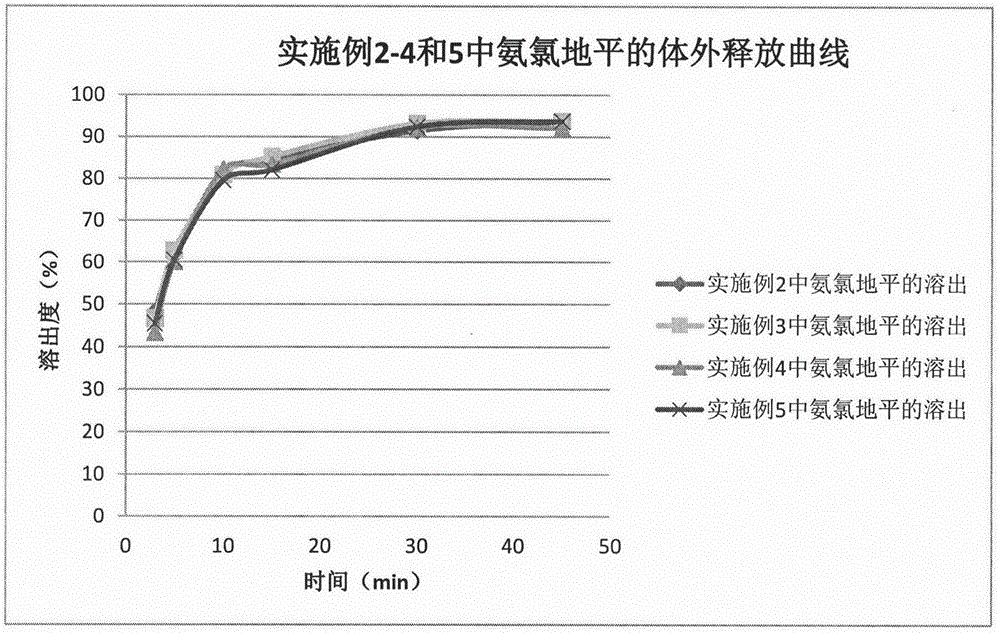
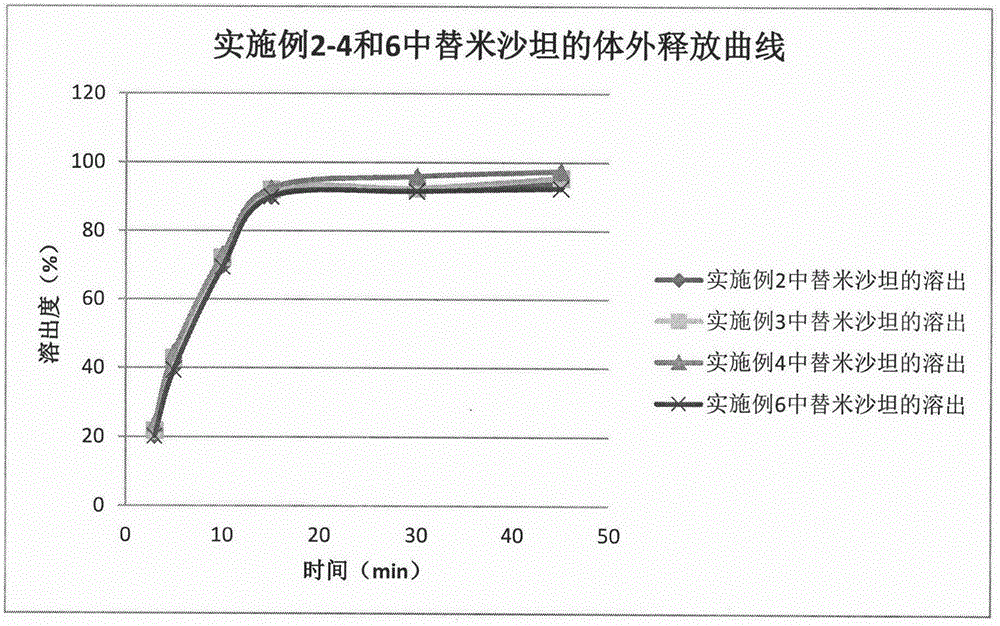
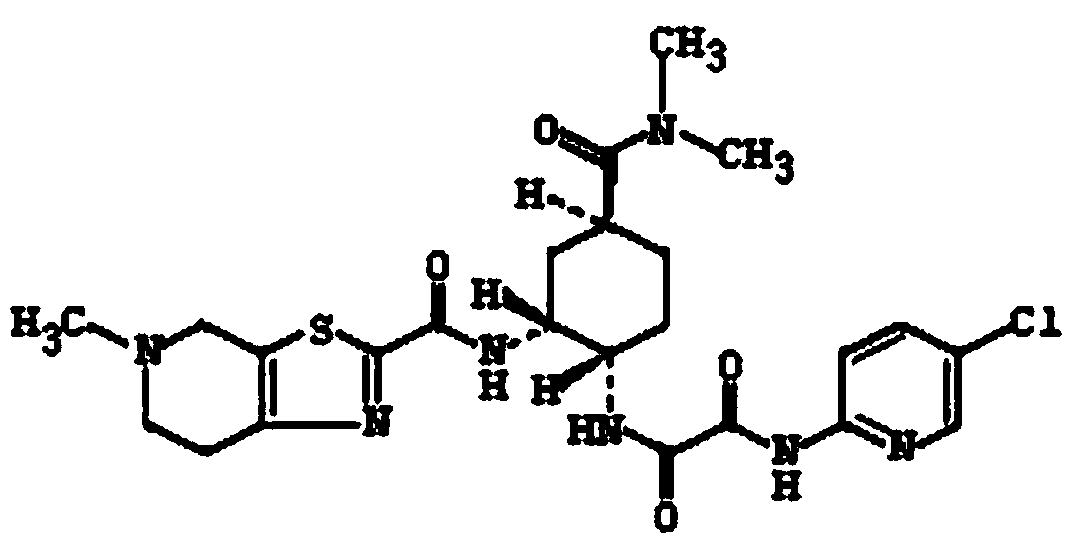
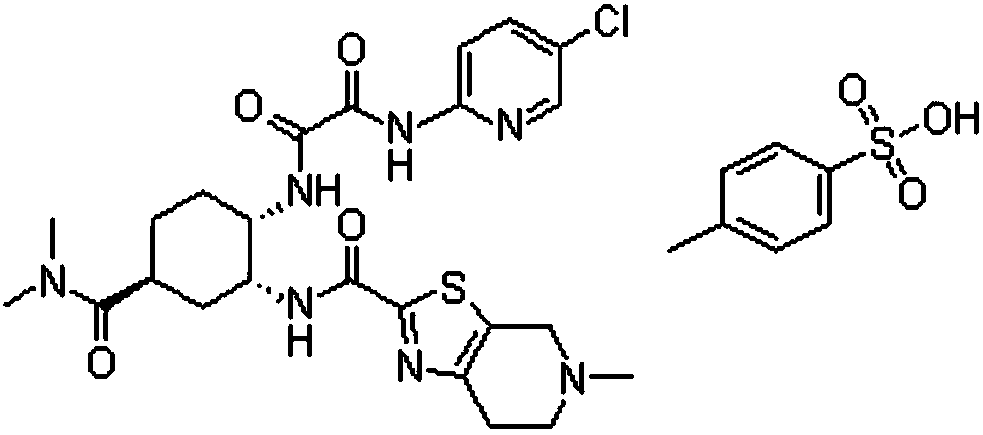
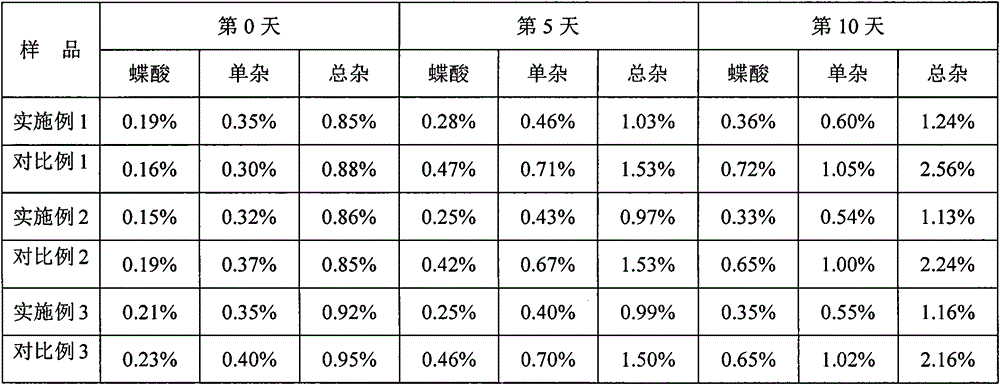
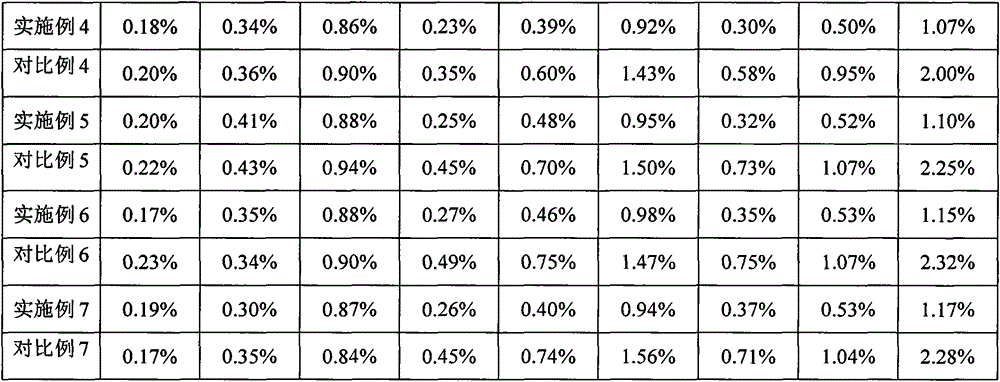
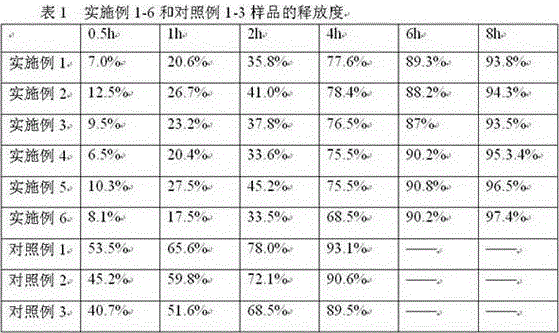
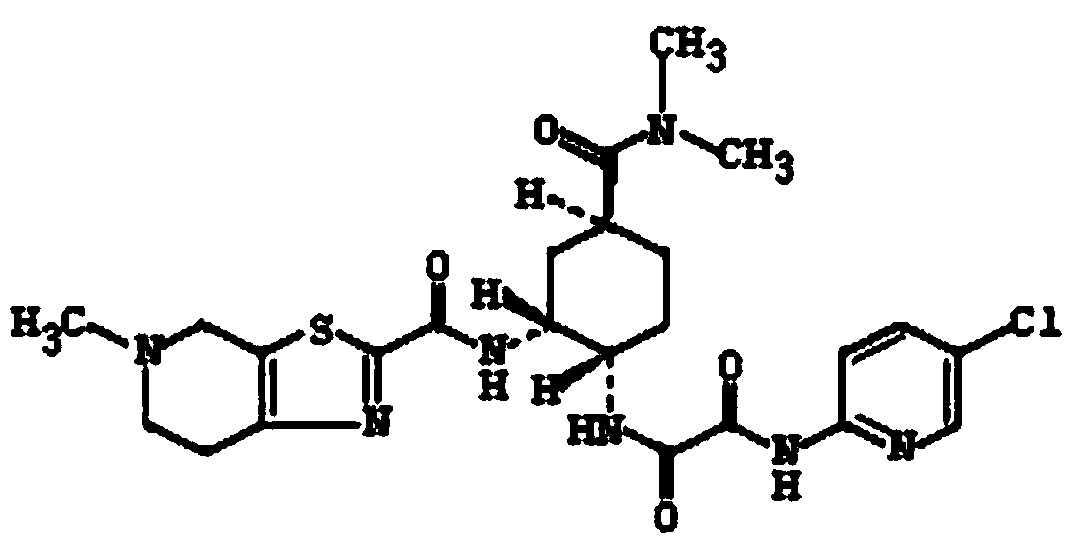
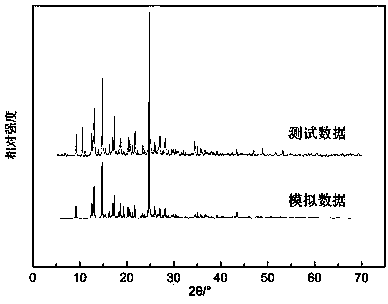
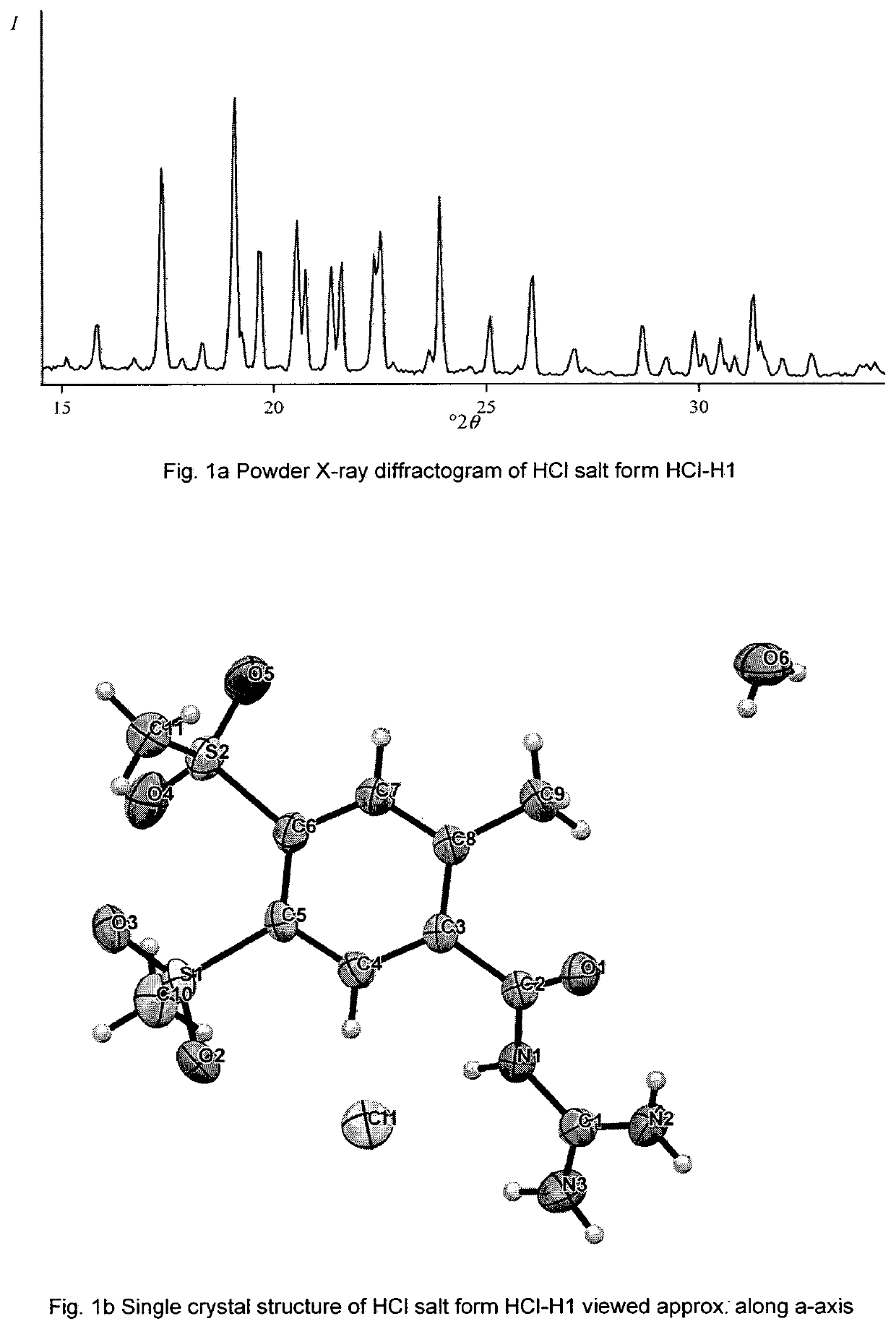
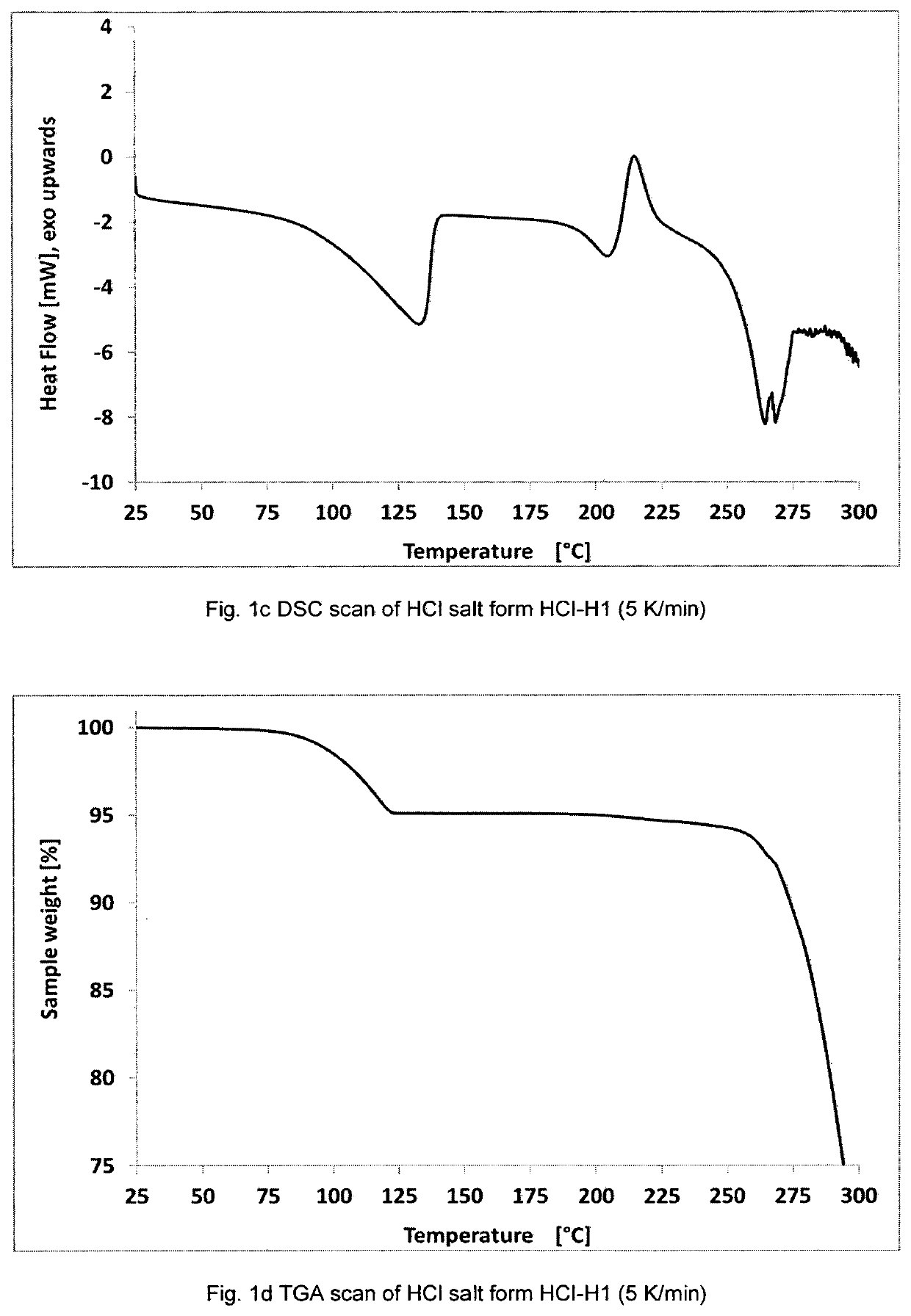
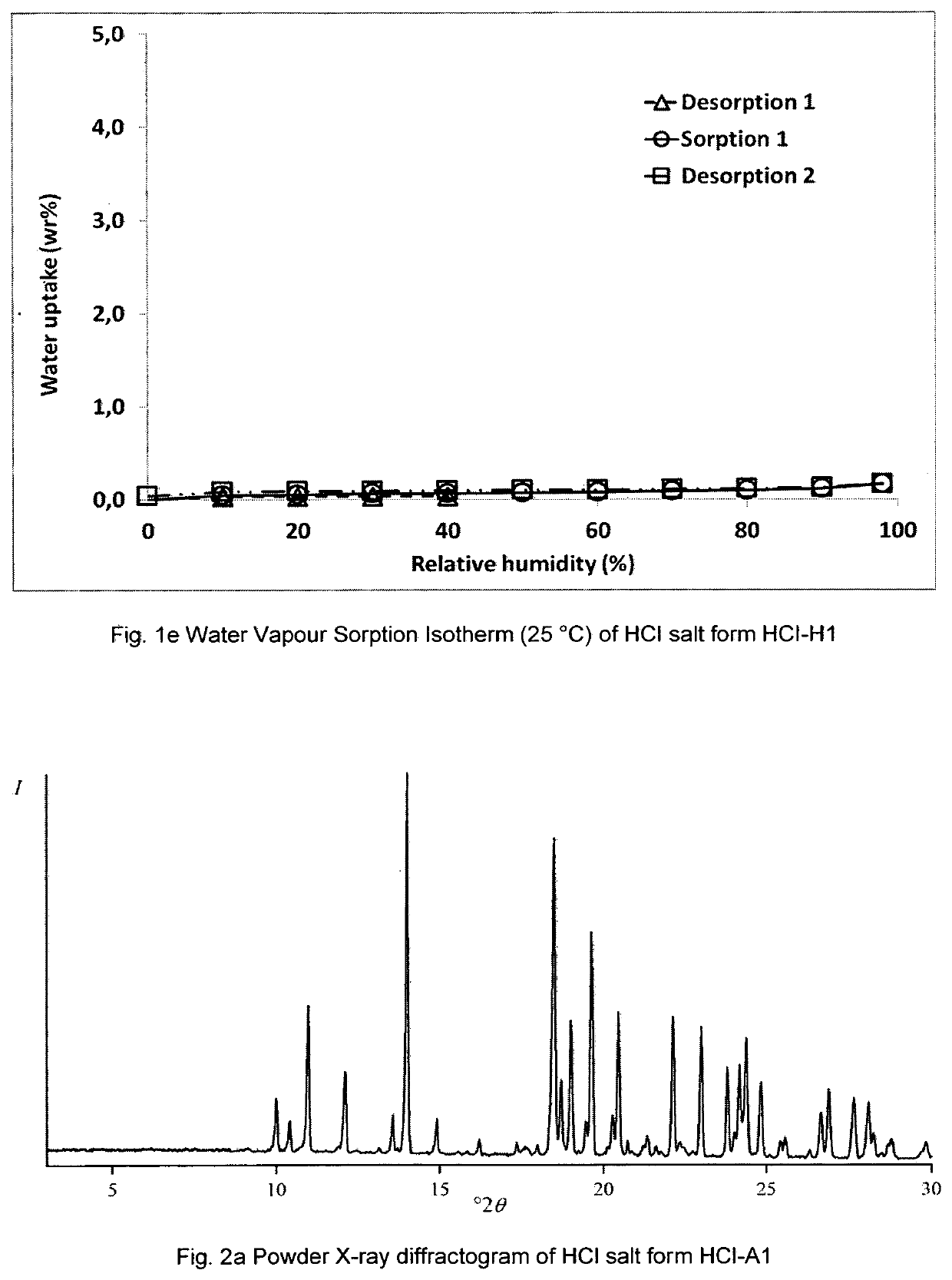
Patents
Literature
51results about How to "Improve dissolution behavior" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
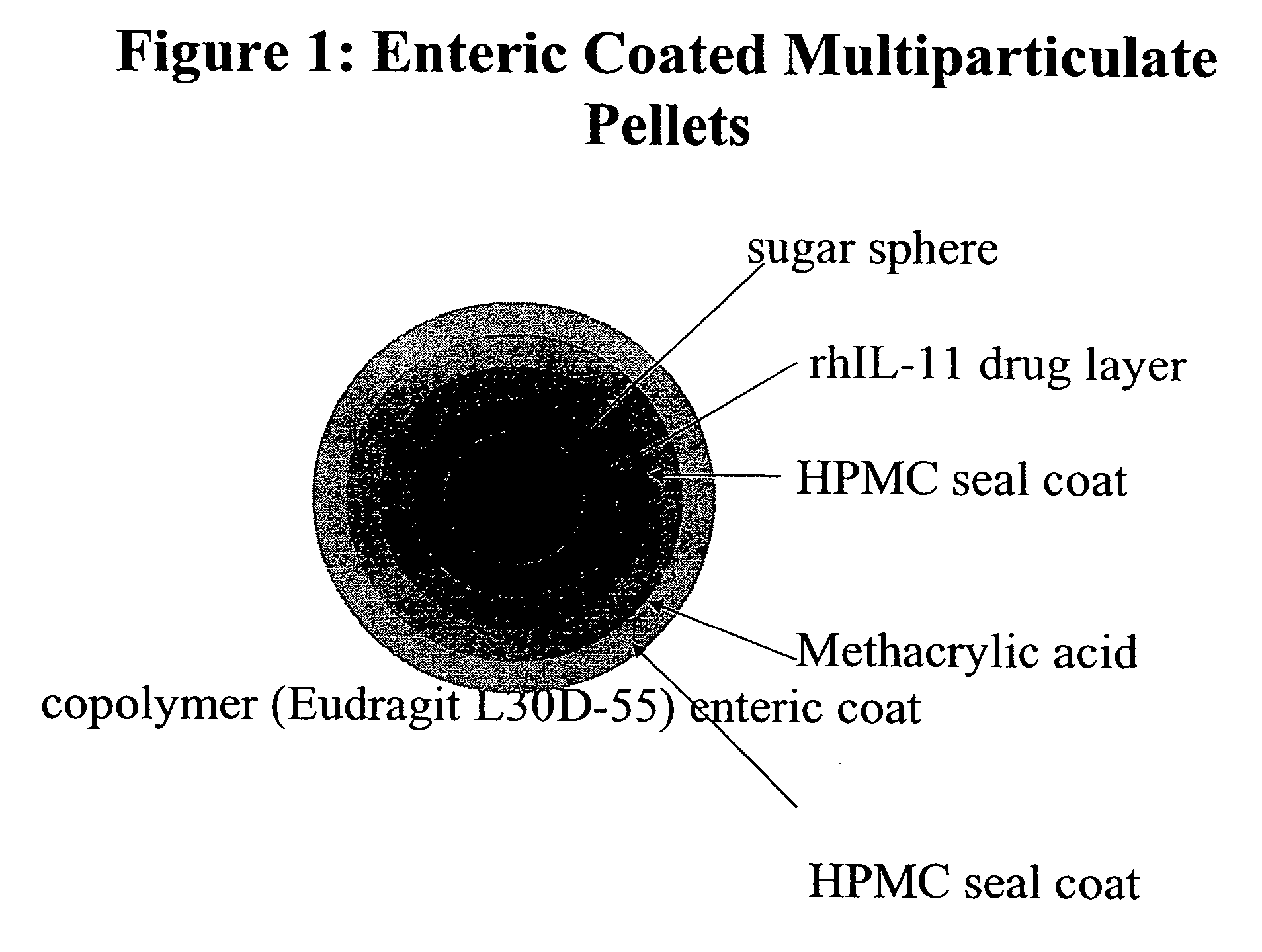
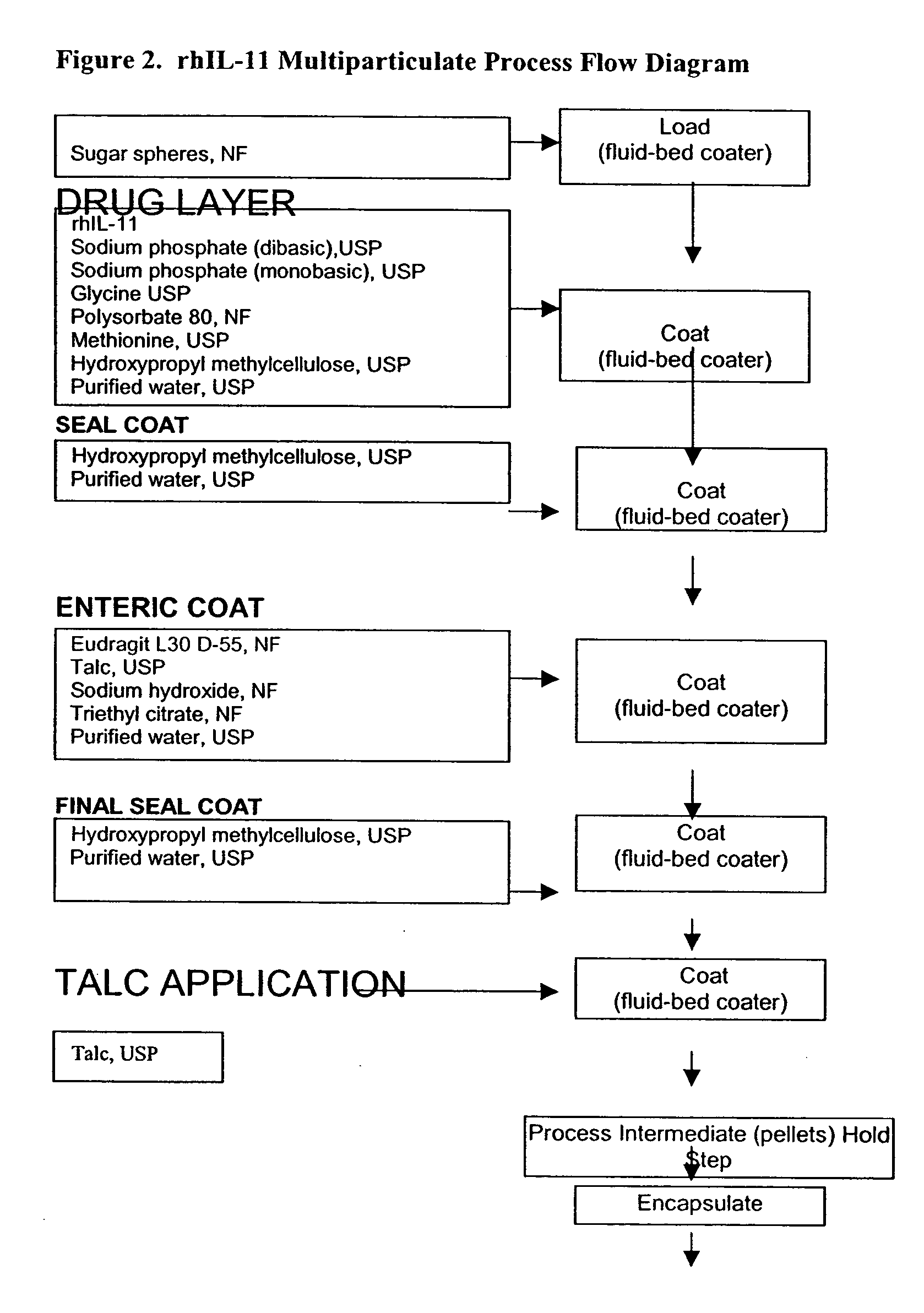
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
InactiveUS20040126358A1Increase ionic strengthReduced strengthAntipyreticAnalgesicsOral medicationWhite blood cell
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
Digestive tract stent with anti-cancer medicinal particles
InactiveCN1792380AFree from pollutionControl drug loadingOrganic active ingredientsSurgeryTopical treatmentTubular stenosis
A scaffold of the granular anticancer medicine for treating the benign or malignant stenosis of esophagus and digestive tract is disclosed, which has 4 structures consisting of scaffold, granular anticancer medicine, selective coated film on the surface of scaffold, and selective external protective layer.
Owner:SHANGHAI JIAO TONG UNIV
Detergent product
InactiveUS20180282673A1Improved shape stabilityHigh dissolution rateFlexible coversWrappersIncrease sizeWater soluble
A detergent product for treating textiles, including a film pouch having a plurality of pouch chambers which are each enclosed by at least one water-soluble film, wherein the pouch chambers are formed by water-soluble films connected to one another in a sealing plane and are separated from one another by sealing sections located in the sealing plane, and wherein the pouch chambers are each filled with a detergent preparation. For intrinsic shape stability of the detergent product and for appearance of the multi-chamber arrangement, a plurality of pouch chambers which are located one after the other are provided in a number n where n≥3, which pouch chambers are arranged as a finite series of pouch chambers with a monotonously increasing size of the footprints of the pouch chambers located in the sealing plane and / or with a monotonously increasing size of the fill volumes of the pouch chambers.
Owner:HENKEL KGAA
Electrochemical etching and polishing of conductive substrates
InactiveUS20110017608A1Minimal undercutGood reproducibilityElectrolysis componentsConductive material chemical/electrolytical removalResistEngineering
A method for electrochemically etching a metal layer through an etch-resist layer pattern using a non-active electrolyte solution is described. The method is particularly useful in fabrication of advanced fuel delivery systems for land-based power generation turbines and aerospace turbine engines; of components for advanced thermal management in aerospace electronic devices and in cooling channels; of stents used in medicine; and of microchannels for sensors, chemical reactors, and dialysis and the like. In one embodiment of the invention the metal layer is copper and the non-active electrolyte solution is a mixture of sodium nitrate and sodium chloride and a pulse electric current is employed to accomplish the electrochemical etching.
Owner:FARADAY TECH INC
Method for producing fine powder and the fine powder produced by the same
ActiveUS9044758B2Inhibit aggregationEasily water-solvable materialsBiocideCarbon compoundsMetallurgyDry ice
Disclosed is a manufacturing method for a fine powder exhibiting improved solubility, little impurity contamination, and a high recovery rate. Material to be ground and a grinding medium are suspended and stirred in a liquefied inert gas dispersion medium such as dried ice, and the material to be ground is made into a sub-micron or nano-sized fine powder. A uniform fine powder can be obtained when the material to be ground is a mixture having two or more components. Impurity contamination can be reduced by using granular dry ice as the grinding medium.
Owner:MORIROKU CHEM
Phosphate tetrahydroberberine drug eutectic and preparation method thereof
InactiveCN106810547AEutectic Solubility EnhancementImprove bioavailabilityAntibacterial agentsOrganic chemistry methodsSpace groupPhosphate
The invention discloses a phosphate tetrahydroberberine drug eutectic and a preparation method thereof. The tetrahydroberberine and phosphate are added and mixed in isopropanol water solution, ethanol water solution or methanol water solution according to the molar ratio; the acquired mixed solution is volatilized under the room temperature. The prepared tetrahydroberberine drug eutectic is a triclinic system, and the space group is P-1, and its axial length (i) is FORMULA; the axial angle is FORMULA. The tetrahydroberberine drug eutectic prepared by the invention inherits the pharmacological activity of tetrahydroberberine, its solubleness, dissolution rate, antibiosis bacillus coli and staphylococcus aureus activities are significantly improved in relative to the tetrahydroberberine, thereby saving drug dosage; the method is good for developing to be drug preparation, and the tetrahydroberberine can be widely applied to the medicine field.
Owner:JIAMUSI UNIVERSITY
Levofloxacin hydrochloride tablets
InactiveCN104288112AImprove dissolution behaviorNo sticky punchAntibacterial agentsOrganic active ingredientsFriabilityTaxifolin
The invention discloses levofloxacin hydrochloride tablets which comprise an active ingredient levofloxacin hydrochloride and medicinal auxiliary materials, wherein the medicinal auxiliary materials comprise a filling agent, a disintegrating agent and a lubricating agent. In the large-scale production of tablets, magnesium stearate generally serves as the lubricating agent, however, because magnesium ion is extremely easily subjected to complexation with levofloxacin or salts thereof, the activity of the medicine is reduced, absorption of gastrointestinal tract on the medicine is interfered, and levofloxacin hydrochloride is unsuitable to be compatible with magnesium stearate. According to the research, when other conventional lubricating agents are used for large-scale preparation in the conventional technology, tests show that the conditions such as severe sticking, bonding, bouncing and low core friability occur in the process of tabletting levofloxacin hydrochloride exist. According to the research in the invention, the lubricating agent adopts talc powder and stearic acid which are added according to a specific compatibility ratio, the lubricating agent has excellent quality controllability in the large-scale production process of the levofloxacin hydrochloride tablets through tests, and the prepared levofloxacin hydrochloride tablets have high safety and effectiveness.
Owner:DIAO GRP CHENGDU PHARMA
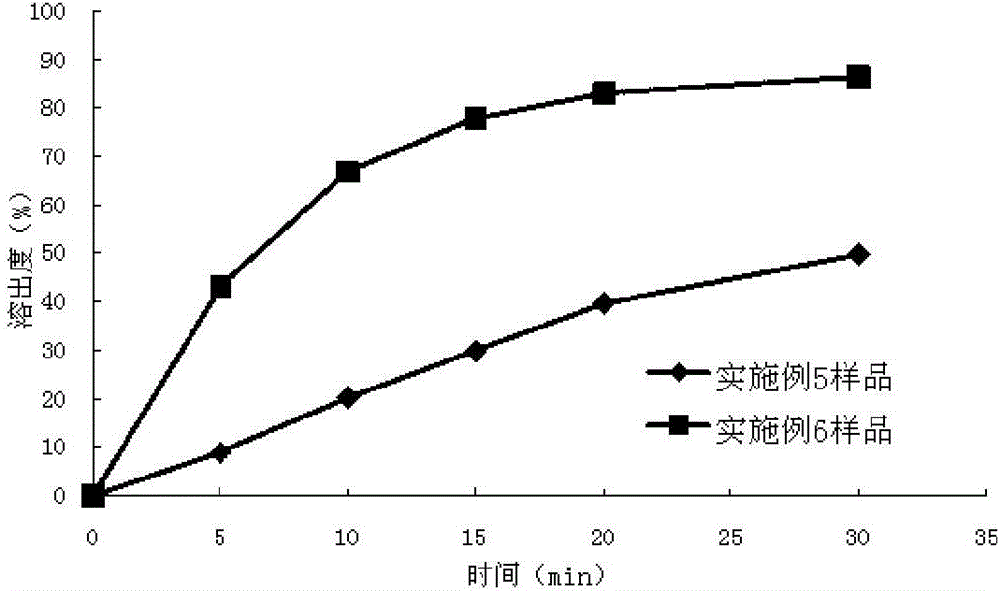
Medicinal composition containing lenvatinib, and preparation method thereof
ActiveCN106551935AImprove uniformityImprove dissolution behaviorInorganic non-active ingredientsAntineoplastic agentsHydrogen phosphateLenvatinib Mesylate
The invention provides a medicinal composition containing lenvatinib. The medicinal composition comprises, by weight, 1-20% of lenvatinib mesylate, 20-60% of anhydrous calcium hydrogen phosphate, 10-55% of a filler, 5-35% of a disintegrating agent, 0-10% of an adhesive and 0.5-5% of a lubricant. Preferably, the particle size of the anhydrous calcium hydrogen phosphate is 200-600 meshes, or the D90 is about 20-75 [mu]m. The medicinal composition has good stability, has a dissolubility reaching 90% or above in 15 min, has effectively improved bioavailability, is easy to prepare, and is suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Montelukast sodium chewable tablet, preparation method and determination method of dissolution rate
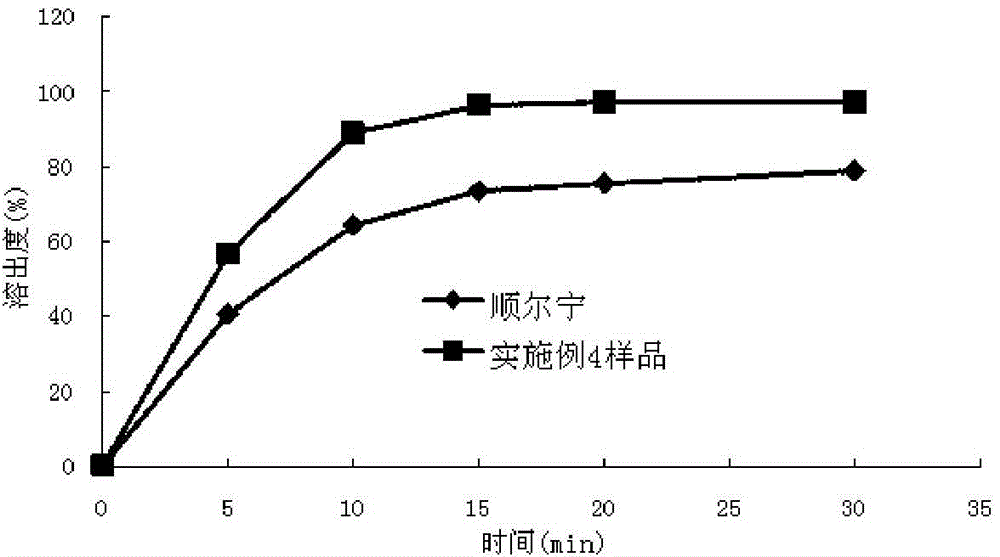
InactiveCN104146975AImprove in vitro dissolutionGuaranteed stabilityAntipyreticComponent separationMANNITOL/SORBITOLDissolution
The invention relates to the field of medicinal preparations and particularly relates to a montelukast sodium chewable tablet, apreparation method and a determination method of a dissolution rate. Raw materials of the montelukast sodium chewable tablet comprise montelukast sodium, mannitol, microcrystalline cellulose, hydroxy propyl cellulose, crosslinking sodium carboxymethylcellulose, aspartame, ferric oxide, magnesium stearate and cherry essence. The hydroxy propyl cellulose is prepared into a solution with a mass percentage content being 6-8% through water being a solvent and a disintegrating agent is prepared through a wet process of an internal addition method. The montelukast sodium chewable tablet is significantly increased in disintegrating speed and in-vitro dissolution rate. By means of a dissolution curve detection method, quality differences among products in each batch can be effectively distinguished. Differences between products in each batch can be reduced better and a quality risk of the products can be controlled in a controllable range.
Owner:BENGBU BBCA MEDICINE SCI DEV
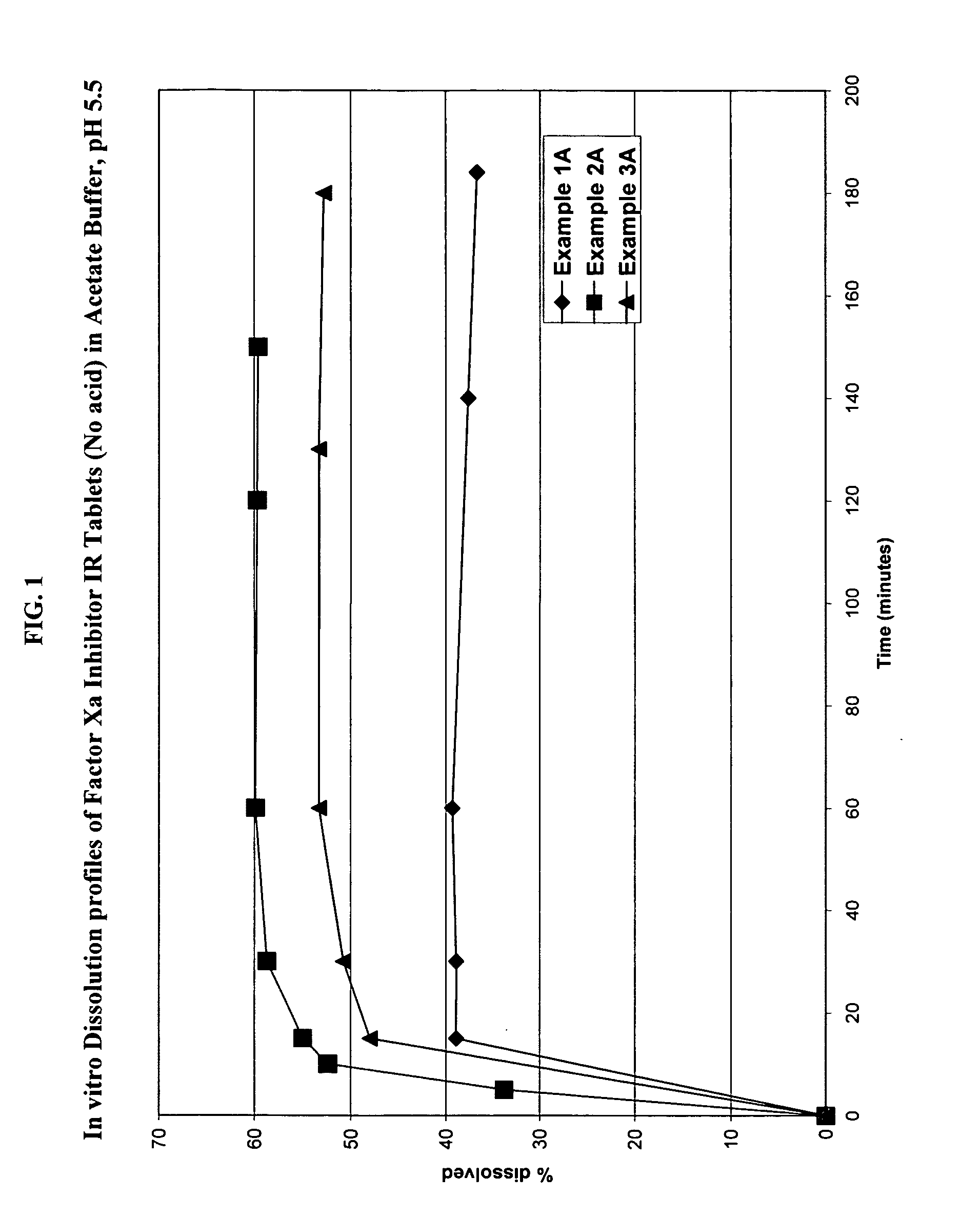
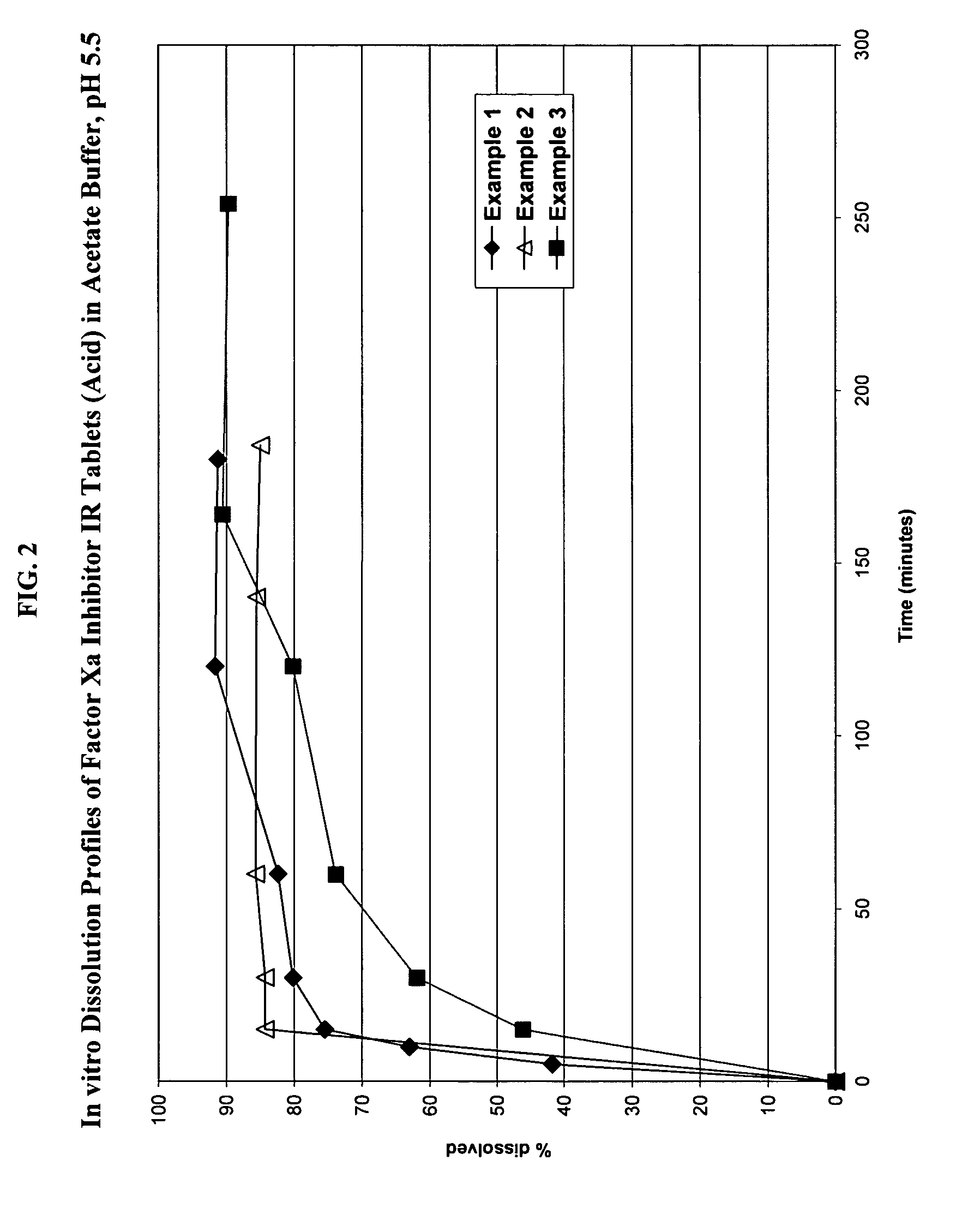
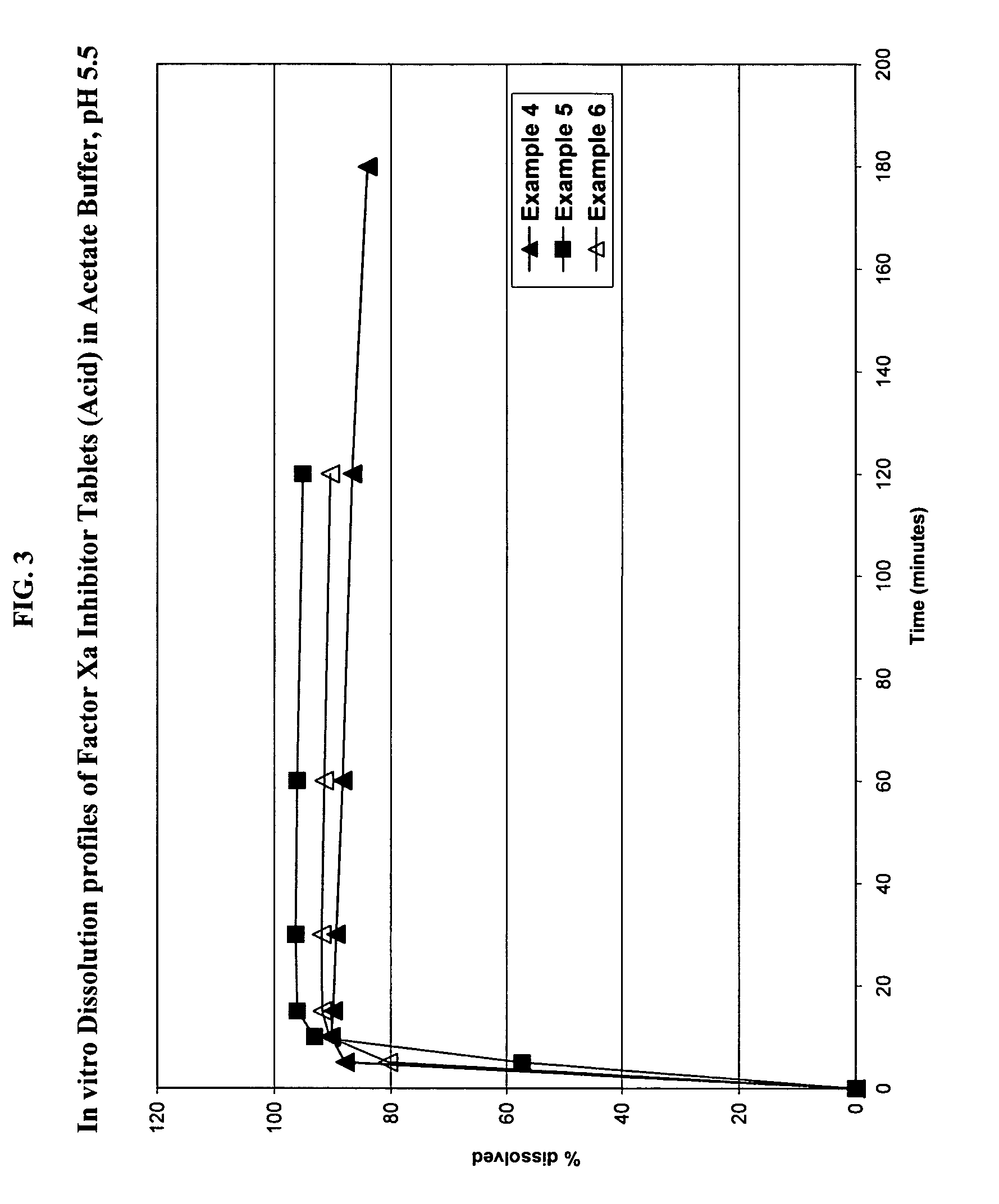
Solid dosage formulation containing a Factor Xa inhibitor and method
InactiveUS20050059719A1Improve oral bioavailabilityLow dissolutionBiocidePill deliverySolubilityCo administration
An oral solid dosage formulation is provided which contains a Factor Xa inhibitor for which oral bioavailability is not reduced by co-administration of antacids, H2 antagonists and proton pump inhibitors. Such solid dosage formulation includes the Factor Xa inhibitor of the structure, a pharmaceutically acceptable carrier, and an acid component, such as tartaric acid, whereby upon ingestion of the oral solid dosage formulation, the acid component increases solubility of the Factor Xa inhibitor in the local environment of the dissolving solid dosage formulation resulting in an otherwise lower degree of supersaturation of the Factor Xa inhibitor in such environment, than if the acid were not present. The result is that precipitation of the Factor Xa inhibitor in the form of its insoluble free base is minimized during dissolution of the Factor Xa inhibitor thereby increasing its oral bioavailability. A method for enhancing bioavailability of the Factor Xa inhibitor is also provided wherein an acid such as tartaric acid is incorporated with the solid dosage pharmaceutical carrier for the Factor Xa inhibitor.
Owner:BRISTOL MYERS SQUIBB CO
Solid preparation of ticagrelor or its pharmaceutically acceptable salt
InactiveCN103860456AImprove dissolution behaviorOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMethyl cellulose
The invention relates to a solid preparation of ticagrelor or its pharmaceutically acceptable salt. Ticagrelor or its pharmaceutically acceptable salt is used as a main drug, lactose, partly pregelatinized starch, hydroxypropyl methyl cellulose, silica and talcum powder are used as auxiliary materials, and powder of the above materials are directly pressed to form a tablet.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Lactate powder and method for the preparation thereof
ActiveUS20150150835A1Improve stabilityGood water solubilityBiocidePowder deliverySodium lactateSODIUM CATION
The present invention relates to a lactate powder, more particularly a lactate powder having a lactate content of at least 20 wt. % and a water content of less than 3.5 wt. %, said powder comprising calcium cations as well as sodium cations. According to the invention, a calcium lactate powder combining high stability with excellent water dissolution properties can be obtained even though anhydrous calcium lactate represents the bulk of the powder if the powder additionally contains a certain amount of sodium lactate. The inventors have found that the presence of sodium lactate greatly improves the dissolution behavior of the anhydrous calcium lactate while maintaining the storage stability of the powder. The present inventors also established that the dissolution behavior of the powder can be further improved by the addition of a fast-dissolving carbohydrate material.
Owner:PURAC BIOCHEM
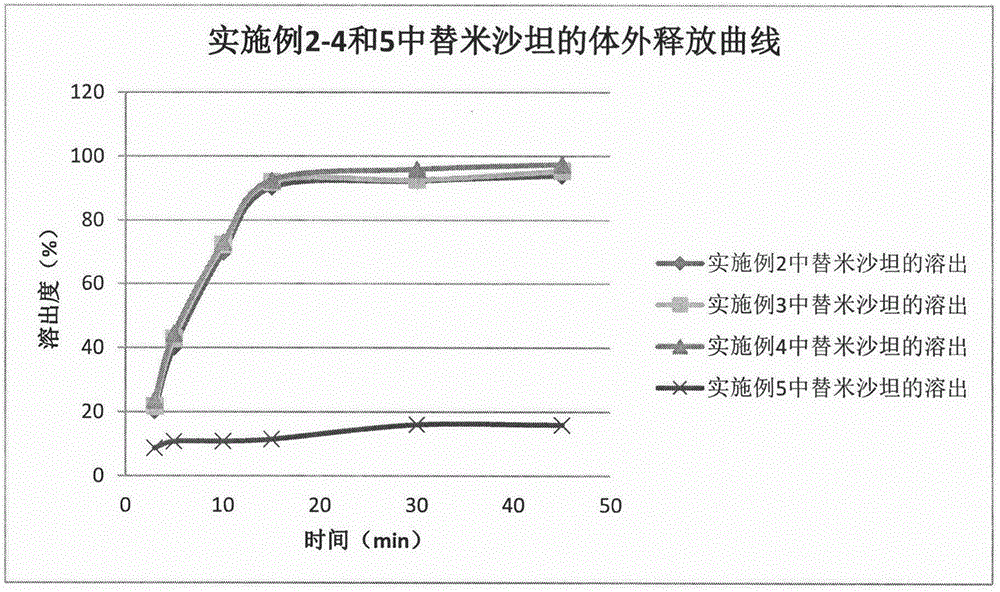
Method for preparing telmisartan and amlodipine double-layer tablets
ActiveCN106822112ASolve the problem of low dissolution rateFix stability issuesOrganic active ingredientsPharmaceutical non-active ingredientsMedicineCurative effect
The invention discloses a method for preparing telmisartan and amlodipine double-layer tablets. The double-layer tablets comprise a telmisartan layer and an amlodipine besylate layer, wherein the telmisartan exists in a solid dispersion form. According to the method for preparing telmisartan and amlodipine double-layer tablets provided by the invention, good dissolution behaviors of the telmisartan and amlodipine besylate are guaranteed, and high stability of the amlodipine besylate is also guaranteed, so that the quality and curative effects of the telmisartan and amlodipine double-layer tablets are ensured. The preparation method is simple and feasible and is suitable for industrial production.
Owner:JIANGSU YABANG AIPUSEN PHARMA
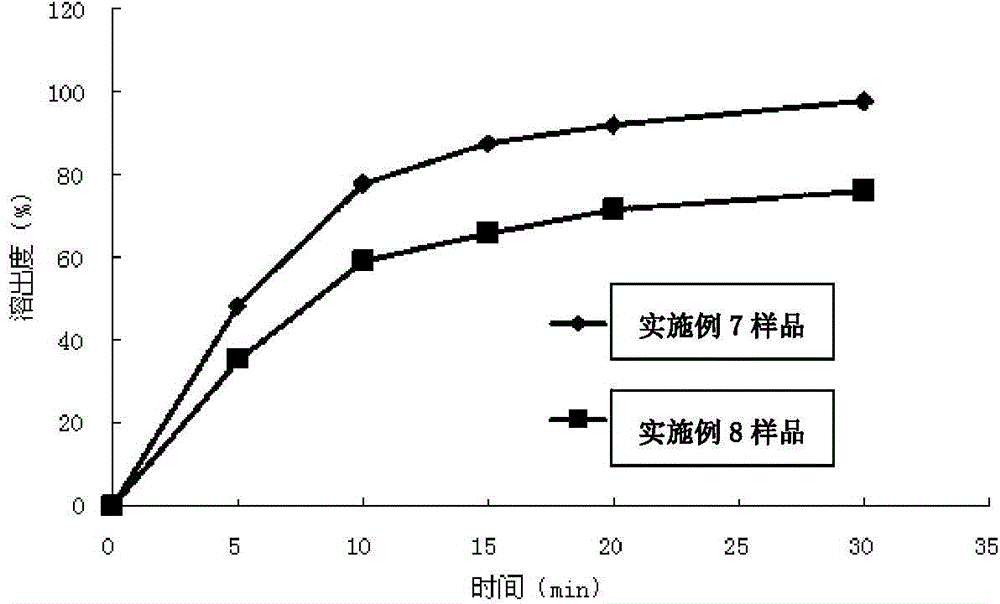
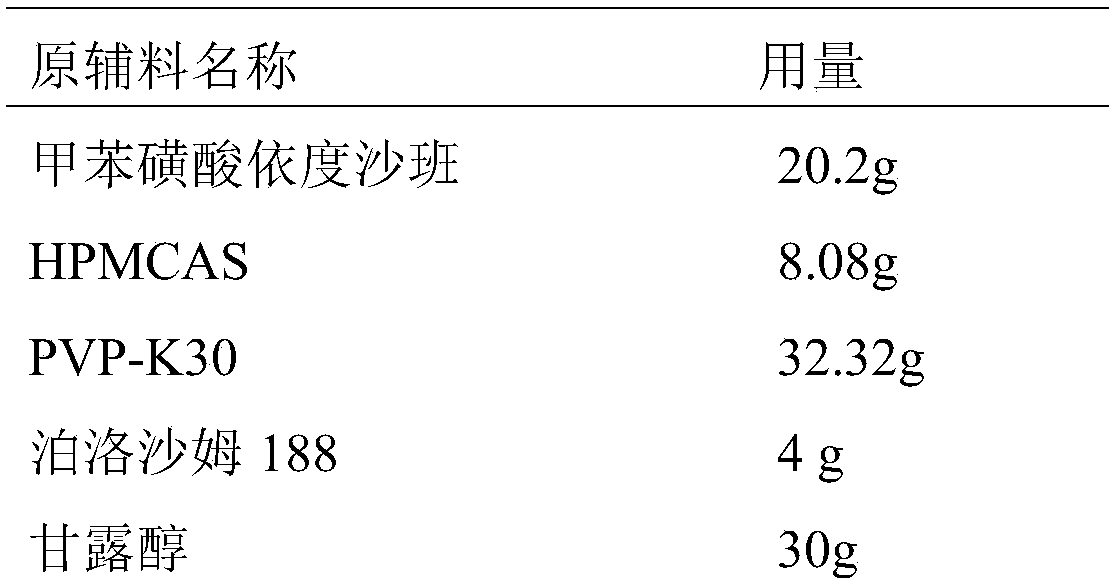
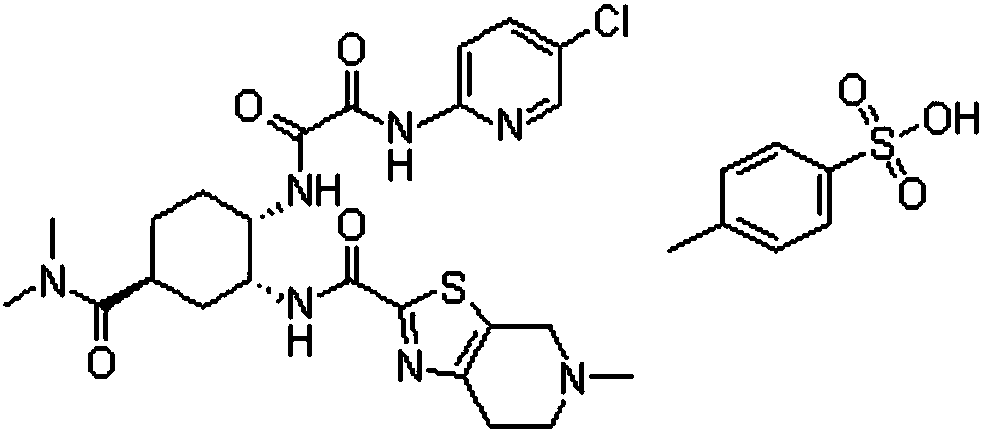
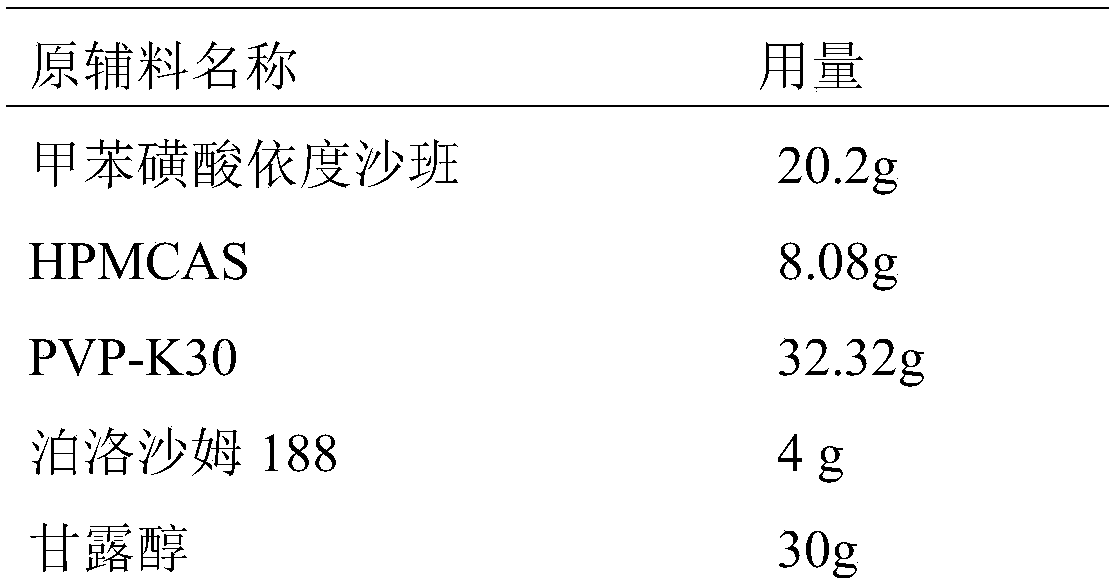
Toluenesulfonic acid edoxaban tablet and preparation method thereof
ActiveCN108743554ALarge specific surface areaImprove wettabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityNeutral ph
The invention discloses a toluenesulfonic acid edoxaban tablet and a preparation method thereof. Raw materials of the toluenesulfonic acid edoxaban tablet include the following substances by mass: 20.2 parts of toluenesulfonic acid edoxaban, 10.1-101 parts of polymeric carrier, 2.02-10.1 parts of surfactant, 30-45 parts of filler and 0.4-0.8 part of lubricant. The polymeric carrier is preferably amixture of PVP K-30 and HPMCAS with the mass ratio of (2-4):1. The preparation method includes dissolving the toluenesulfonic acid edoxaban, the polymeric carrier and the surfactant in a solvent; spraying and drying to obtain a solid dispersion of the toluenesulfonic acid edoxaban; 2) mixing the solid dispersion of the toluenesulfonic acid edoxaban with other accessories and conducting tabletting. The tablet can significantly improve the dissolution rate and the solubility of a drug, increase the drug absorption and improve the bioavailability. In particular, the tablet significantly improvesthe dissolution behavior in a buffer solution of neutral pH 6.8 compared with the existing preparations.
Owner:安徽佳和药业有限公司
Solid preparation of clopidogrel or pharmaceutically acceptable salt thereof
ActiveCN101926756AAvoid water degradationShort production cycleOrganic active ingredientsPharmaceutical delivery mechanismMethyl cellulosePharmaceutical preservatives
The invention relates to a solid preparation of clopidogrel or a pharmaceutically acceptable salt thereof, and the adopted technical scheme is as follows: taking the clopidogrel or the pharmaceutically acceptable salt as a principal agent, taking lactose, partially pregelatinized starch, hydroxypropyl methyl cellulose, silicon dioxide and talcum powder as excipients, and directly pressing powder into tablets.
Owner:CHINA RESOURCES SAIKE PHARMA
Pharmaceutical preparation comprising cyclin inhibitor and preparation method thereof
InactiveUS20180280392A1Suitable for medical applicationImprove dissolution behaviorOrganic active ingredientsCapsule deliveryKetoneCyclin
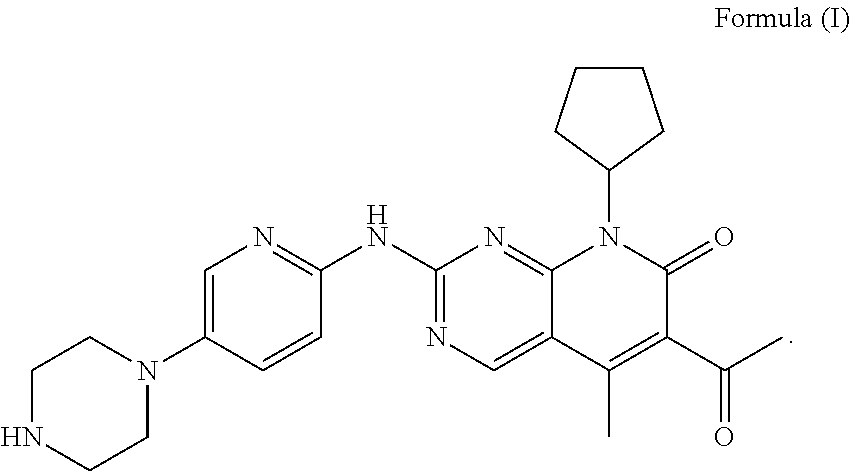
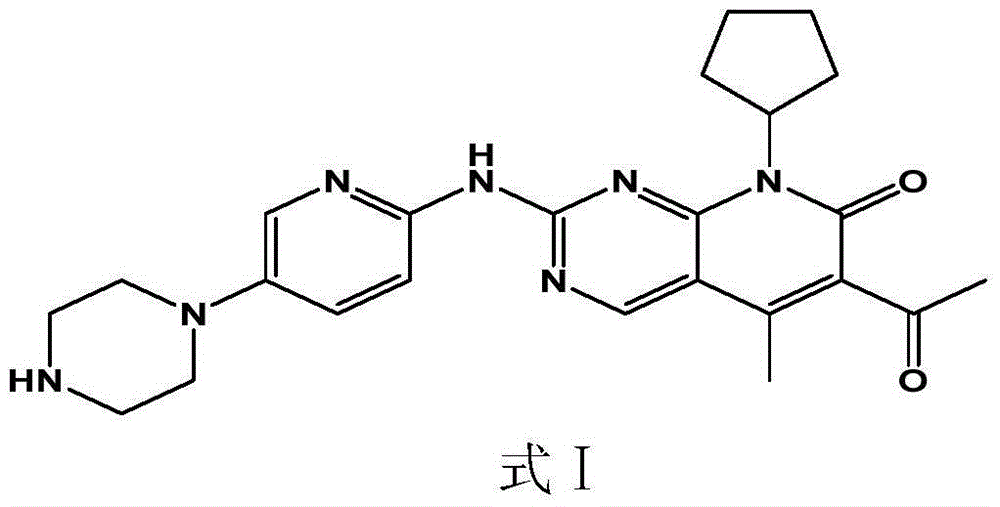
Disclosed is a pharmaceutical preparation having 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazine-1-yl-pyridine-2-yl amino group)-8H-pyrido[2,3-d]pyrimidine-7-one or salt thereof as an active ingredient, the salt comprising hydrochloride or isethionate, and the dosage form thereof comprising tablets and capsules both having good stability and excellent dissolution performance.
Owner:JIANGSU HANSOH PHARMA CO LTD
Repaglinide and metformin hydrochloride tablet pharmaceutical composition and preparation method thereof
ActiveCN105663131AGood dissolution behaviorGood for absorptionOrganic active ingredientsMetabolism disorderMetformin HydrochlorideWater soluble
The invention provides a repaglinide and metformin hydrochloride tablet pharmaceutical composition; in a prescription, the amount of a disintegrating agent is greatly reduced compared with the prior art; in a process, microcrystalline cellulose particles are prepared in advance, repaglinide is made into a solution, repaglinide particles are prepared on the microcrystalline cellulose particles through fluidized bed atomizing, and the repaglinide particles are mixed and tabletted with metformin hydrochloride particles. The prepared repaglinide and metformin hydrochloride tablet product is stable, the insoluble drug repaglinide and the water-soluble drug metformin hydrochloride are both released synchronously according to requirements, and the bioavailability is better compared with that of the prior art; and at the same time, the problem of uneven mixing of the low-dosage drug repaglinide is solved, and the drug efficacy playing is greatly promoted.
Owner:CHENGDU EASTON BIOPHARM CO LTD
Male health composition containing folic acid and zinc gluconate, and preparation method of preparations thereof
ActiveCN106177964AReduce adverse reactionsIdeal generationOrganic active ingredientsInorganic non-active ingredientsSodium bicarbonateMedicine
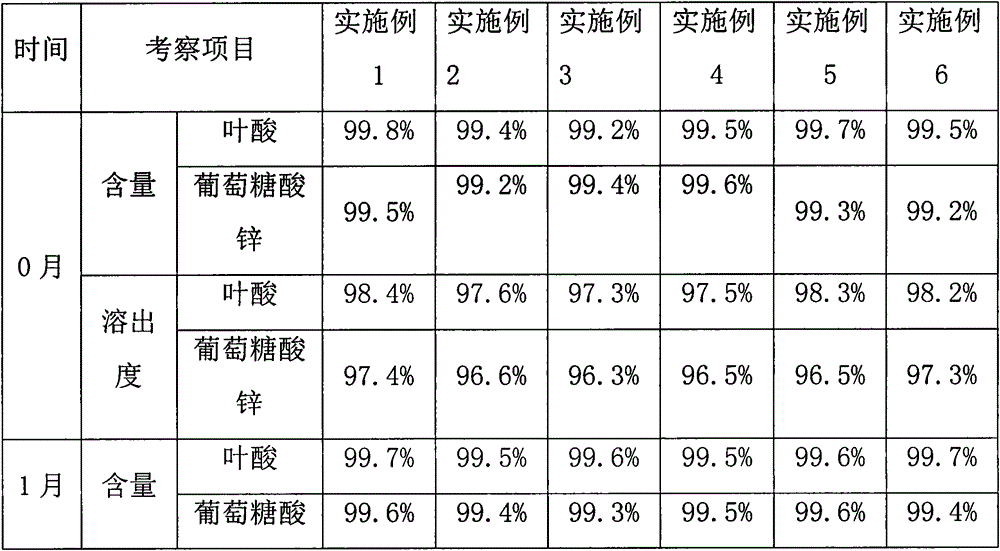
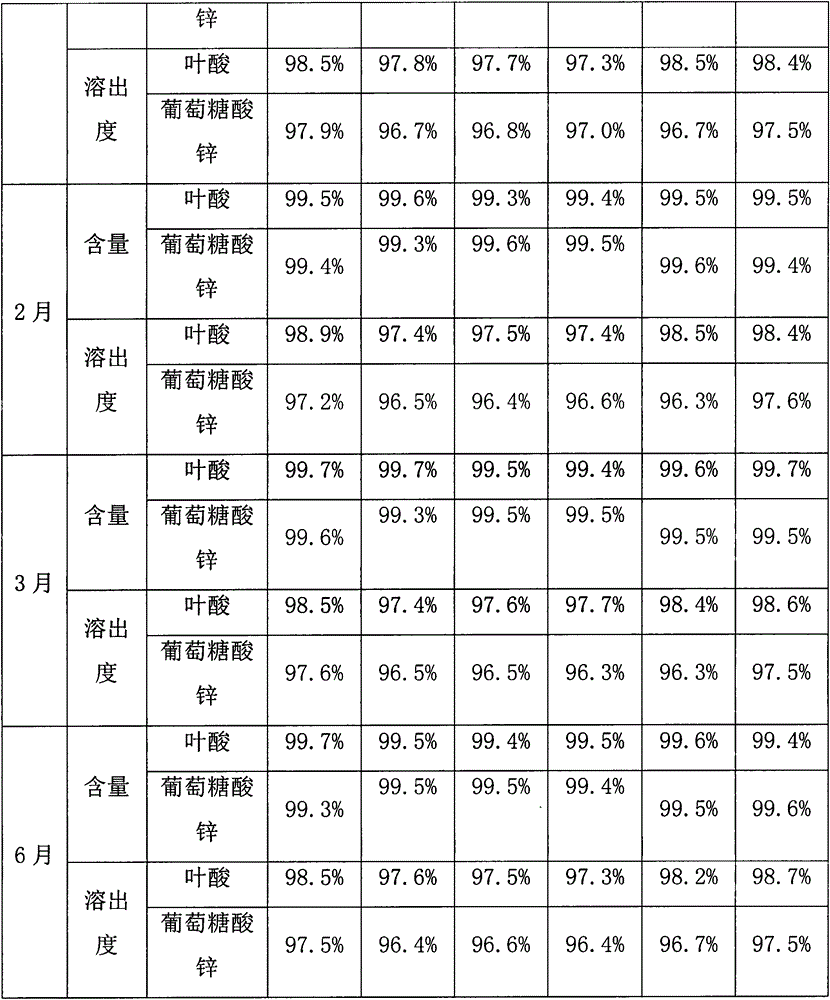
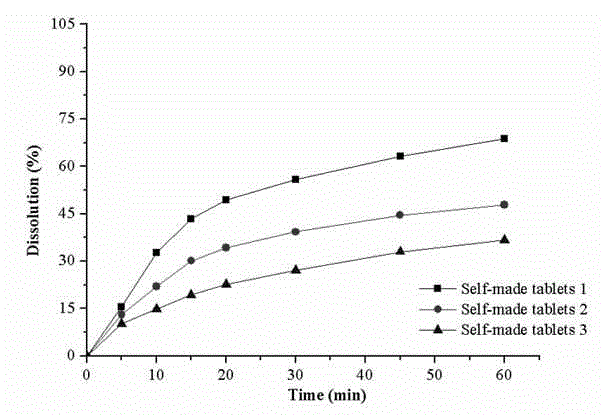
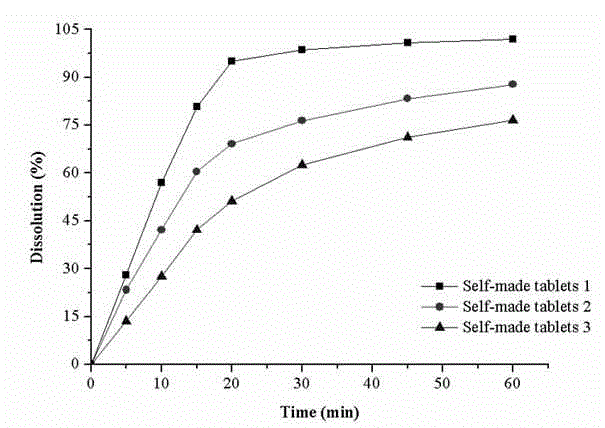
The invention provides a male health composition containing folic acid and zinc gluconate, and a preparation method of preparations thereof. The health composition contains folic acid and zinc gluconate, and also contains sodium bicarbonate and a pharmaceutically acceptable auxiliary material; and a weight part ratio of folic acid to zinc gluconate to sodium bicarbonate is 0.2-0.8:35-70:100-500. The health composition can be used for preparing oral solid preparations not limited to a tablet, a capsule and a powder, improves the dissolution rate and the dissolution degree of folic acid and zinc gluconate, substantially reduces zinc gluconate induced gastrointestinal bad reaction, and improves the bioavailability of zinc gluconate.
Owner:北京斯利安药业有限公司
Preparation and application of drug powder
ActiveCN104606139AGood dispersionInhibit aggregationPowder deliveryOrganic active ingredientsMicrometerDissolution
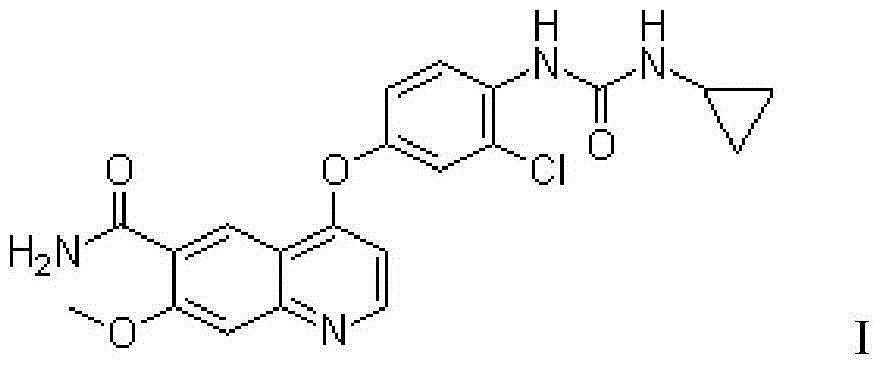
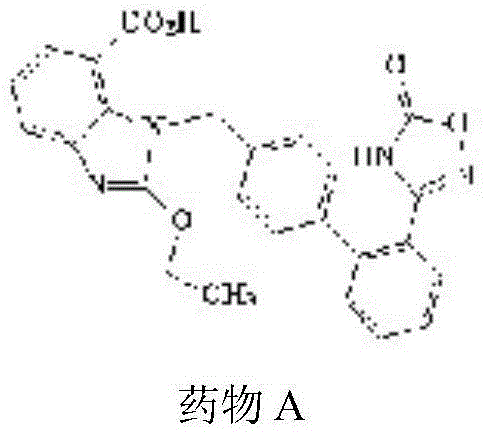
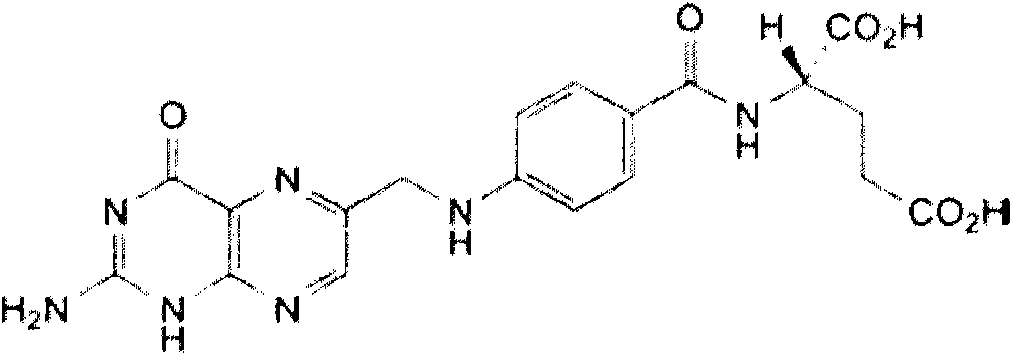
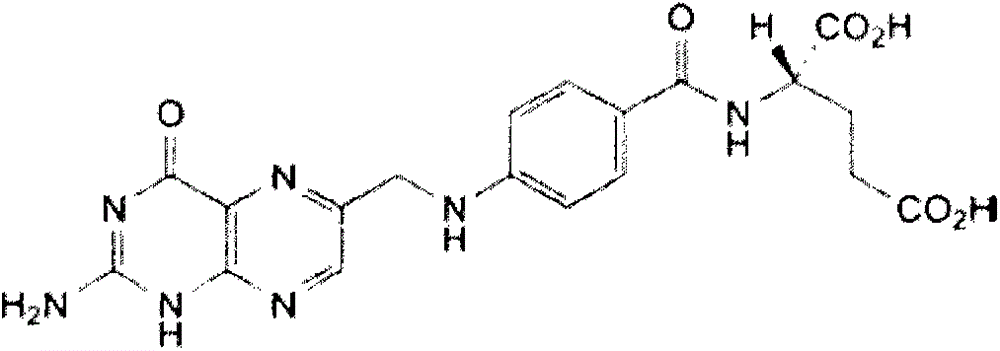
Belonging to the field of pharmaceutical preparations, the invention provides a preparation method for a drug A (formula 1) powder and its application in oral solid preparations. The method includes: dispersing the crude drugs of drug A in a hydrophilic suspending aid solution, employing wet grinding to reduce the particle size to less than 5 micrometers, adding a spray-drying support agent, and combining spray drying, thus obtaining the drug A powder with an average particle size of less than 20 micrometers. The drug A powder prepared by the invention has high surface hydrophilicity, small particle size and good redispersibility in water. Compared with the crude drugs, the oral solid preparations prepared from the drug A powder and suitable auxiliary materials can significantly improve the dissolution rate. The preparation method for the drug A powder provided by the invention is suitable for industrial production and has high application value. (formula I).
Owner:SHENYANG PHARMA UNIVERSITY +2
Pharmaceutical preparation containing cyclin inhibitor, and preparation method thereof
InactiveCN105616418AImprove dissolution behaviorImprove bioavailability in vivoOrganic active ingredientsCapsule deliverySulfonatePharmaceutical drug
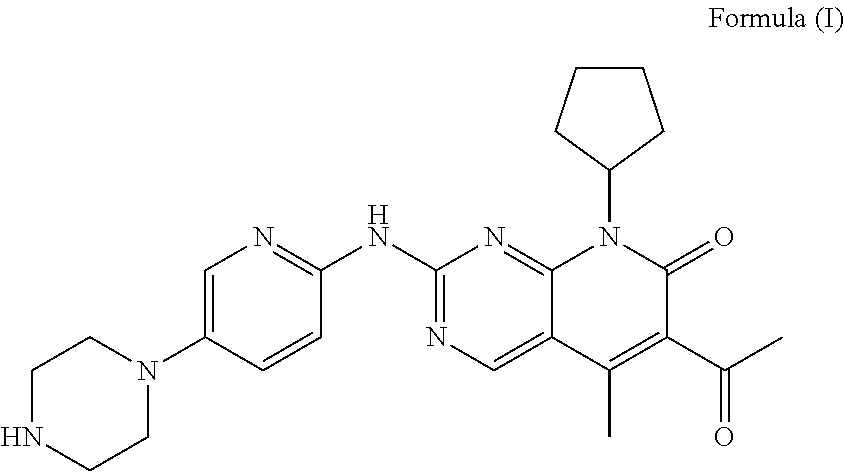
The present invention discloses a pharmaceutical preparation containing a cyclin inhibitor, and a preparation method thereof, and particularly relates to a pharmaceutical preparation adopting 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazine-1-yl-pyridine-2-yl-amino)-8H-pyrido[2,3-d]pyrimidine-7-one represented by a formula I or a salt thereof as an active component, wherein the salt comprises a hydrochloride and an isethionate, and the dosage form of the pharmaceutical preparation comprises tablets and capsules, and has good stability and excellent dissolution behavior. The formula I is defined in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Dipfluzine-p-hydroxybenzoic acid eutectic crystal and preparation method thereof
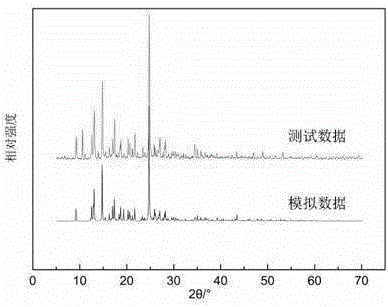
InactiveCN105601589AChange physical and chemical propertiesImprove solubilityOrganic compound preparationOrganic chemistry methodsSolubilityDiffusion methods
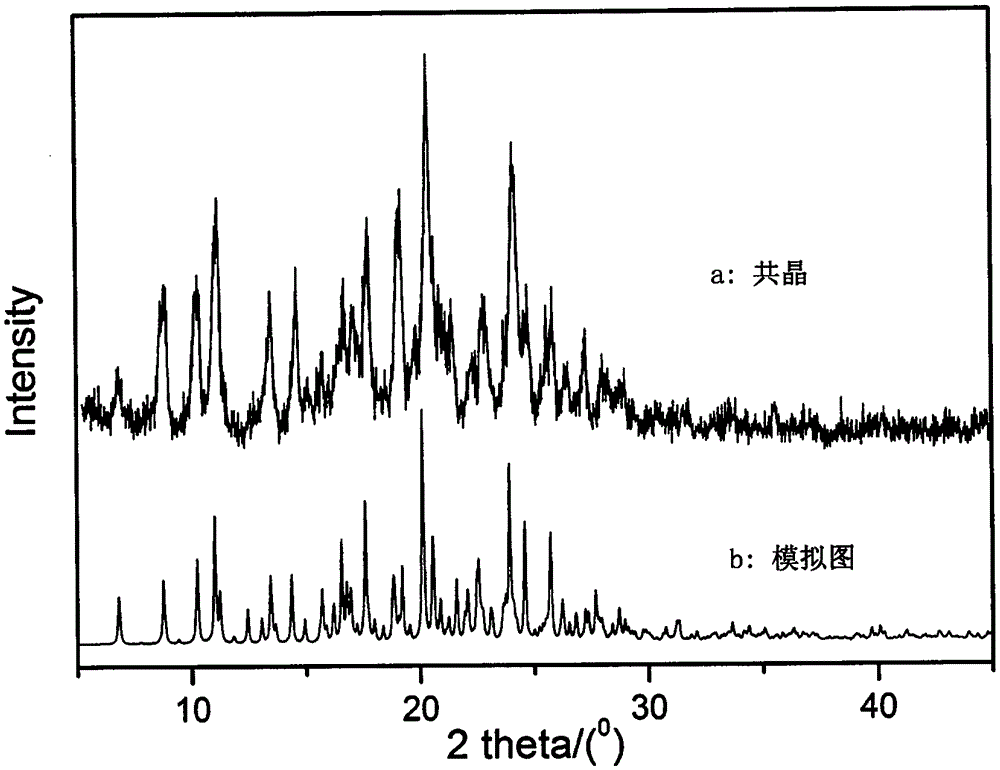
The invention discloses a novel dipfluzine medicine eutectic crystal and a preparation method thereof. According to the preparation method, a dipfluzine raw medicine is selected as medicine API, p-hydroxybenzoic acid is selected as a medicine precursor, the prepared dipfluzine medicine eutectic crystal is of a monoclinic system, and the space group is P2(1), wherein a is equal to 26.150(9) angstroms, b is equal to 10.936(3) angstroms, c is equal to 11.963(3) angstroms, beta is equal to 97.49(1) degrees, V is equal to 3391.95(396) cubic angstroms, and z is equal to 2. A vapor diffusion method is utilized in the preparation process of the medicine eutectic crystal, a selected small-cup solvent is a mixed solvent of ethanol and water, a selected big-cup solvent is acetonitrile, and dipfluzine has different solubilities in the selected solvents, so that crystals can be separated in the volatile transfer process of the solvents. The dipfluzine-p-hydroxybenzoic acid eutectic crystal disclosed by the invention is different from X-ray powder diffraction, DSC and infrared spectrum of a physical mixture of dipfluzine and p-hydroxybenzoic acid crystals, and the solubility and dissolving behavior of the medicine eutectic crystal prepared by virtue of the preparation method are obviously improved.
Owner:HEBEI MEDICAL UNIVERSITY
Detergent product
ActiveUS10745653B2Adverse effect on the dissolution behavior of the film pouchReduce mechanical contactFlexible coversWrappersPolymer scienceSoluble Film
A detergent product for treating textiles, in particular for cleaning textiles and / or washing textiles, is illustrated and described, including a film pouch having at least one pouch chamber which is enclosed by at least one water-soluble film and contains a detergent preparation. In order to improve the dissolution behavior of the detergent product, the film pouch has at least one through-flow opening arranged outside of the pouch chamber.
Owner:HENKEL KGAA
A kind of folic acid solid dispersion and preparation method thereof
ActiveCN105395554BGood content uniformityImprove bioavailability in vivoOrganic active ingredientsNervous disorderPolyethylene glycolDissolution
Owner:北京斯利安药业有限公司
Folic acid solid dispersion body and preparation method thereof
ActiveCN105395554AGood content uniformityImprove bioavailability in vivoOrganic active ingredientsPharmaceutical non-active ingredientsMANNITOL/SORBITOLPolyethylene glycol
The present invention relates to the field of medicines, particularly to a folic acid solid dispersion body and a preparation method thereof, wherein the solid dispersion body comprises a hydrophilic carrier and folic acid, a weight ratio of the folic acid to the hydrophilic carrier is 1:5-1:50, and the hydrophilic carrier is one or a composition comprising one or a plurality of materials selected from poloxamer, polyethylene glycol, povidone and mannitol. According to the present invention, the particle size distribution of the folic acid solid dispersion body is 80-150 mesh; the preparation method is a melting method or a grinding method; the clinical preparation form of the solid dispersion body is an oral solid preparation, and the 30 min dissolution of the oral solid preparation is not less than 90%; and the folic acid solid dispersion body of the present invention has comprehensive advantages of uniform content, good dissolution, high bioavailability and good stability, and the characteristics of environmental protection and easy industrialization achieving are provided.
Owner:北京斯利安药业有限公司
Mirabegron composition
InactiveCN106176650AImprove dissolution behaviorReduce releaseOrganic active ingredientsPharmaceutical non-active ingredientsActive matterPolyethylene glycol
The invention relates to a mirabegron composition and a preparation method thereof and belongs to the technical field of pharmacy. According to the technical scheme, the mirabegron sustained-release composition is characterized in that every 1000 tablets comprise 25-50 g of mirabegron, 15-45 g of hydroxypropyl methylcellulose (K4M), 0.15-0.3 g of butylated hydroxytoluene, 50-160 g of polyoxyethylene (POLYOX N80), 10-32 g of polyethylene glycol 6000, 0.8-1.4 g of magnesium stearate and 8-15 g of powdery silicon dioxide. Through implementation of the technical scheme, a clinic medicine which is excellent in dissolution behavior is obtained. Due to the adding of silicon dioxide, the sustained release of active matter mirabegron is performed to some extent.
Owner:DISHA PHARMA GRP +1
A kind of agomelatine tablet and preparation method thereof
ActiveCN104523639BImprove dissolution behaviorImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsGluconic acidDissolution
The invention discloses an agomelatine tablet of which the formula comprises the following components by weight: 25g of agomelatine crystal form II, 55-70g of lactose, 13-30g of starch, 6-12g of povidone K30, 5-10g of carboxymethyl starch sodium, 1-3g of stearic acid, 0.5-1.5g of magnesium stearate, 0.1-0.5g of silicon dioxide, 4-6g of coating powder, 70-90g of 75% ethanol, 1-2g of potassium gluconate, 1-2g of arabic gum and 1-2g of magnesium citrate. The potassium gluconate, Arabic gum and magnesium citrate are added into the formula of the agomelatine tablet provided by the invention to adjust the dissolution rate of the tablet, so that the coated tablet has good dissolution behavior and stronger stability.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD
A kind of edoxaban tosylate tablet and preparation method thereof
ActiveCN108743554BLarge specific surface areaImprove wettabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityNeutral ph
The invention discloses a toluenesulfonic acid edoxaban tablet and a preparation method thereof. Raw materials of the toluenesulfonic acid edoxaban tablet include the following substances by mass: 20.2 parts of toluenesulfonic acid edoxaban, 10.1-101 parts of polymeric carrier, 2.02-10.1 parts of surfactant, 30-45 parts of filler and 0.4-0.8 part of lubricant. The polymeric carrier is preferably amixture of PVP K-30 and HPMCAS with the mass ratio of (2-4):1. The preparation method includes dissolving the toluenesulfonic acid edoxaban, the polymeric carrier and the surfactant in a solvent; spraying and drying to obtain a solid dispersion of the toluenesulfonic acid edoxaban; 2) mixing the solid dispersion of the toluenesulfonic acid edoxaban with other accessories and conducting tabletting. The tablet can significantly improve the dissolution rate and the solubility of a drug, increase the drug absorption and improve the bioavailability. In particular, the tablet significantly improvesthe dissolution behavior in a buffer solution of neutral pH 6.8 compared with the existing preparations.
Owner:安徽佳和药业有限公司
Tetrahydroberberine phosphate drug co-crystal and preparation method thereof
InactiveCN106810547BEutectic Solubility EnhancementImprove bioavailabilityAntibacterial agentsOrganic chemistry methodsSpace groupPhosphate
The invention discloses a phosphate tetrahydroberberine drug eutectic and a preparation method thereof. The tetrahydroberberine and phosphate are added and mixed in isopropanol water solution, ethanol water solution or methanol water solution according to the molar ratio; the acquired mixed solution is volatilized under the room temperature. The prepared tetrahydroberberine drug eutectic is a triclinic system, and the space group is P-1, and its axial length (i) is FORMULA; the axial angle is FORMULA. The tetrahydroberberine drug eutectic prepared by the invention inherits the pharmacological activity of tetrahydroberberine, its solubleness, dissolution rate, antibiosis bacillus coli and staphylococcus aureus activities are significantly improved in relative to the tetrahydroberberine, thereby saving drug dosage; the method is good for developing to be drug preparation, and the tetrahydroberberine can be widely applied to the medicine field.
Owner:JIAMUSI UNIVERSITY
Crystalline modifications of N-(4,5-bismethanesulfonyl-2-methylbenzoyl)guanidine hydrochloride and N-(4,5-bismethanesulfonyl-2-methylbenzoyl)guanidine salts
ActiveUS10981864B2Improve dissolution behaviorHigh crystallinityNervous disorderMetabolism disorderMethyl benzeneAcyl group
The present invention relates to novel crystalline modifications of N-(4,5-bismethanesulfonyl-2-methylbenzoyl)guanidine hydrochloride, novel N-(4,5-bismethanesulfonyl-2-methylbenzoyl)guanidine salts and their crystalline modifications and processes of manufacturing and pharmaceutical formulations thereof.
Owner:MERCK PATENT GMBH
Desloratadine citrate disodium capsules as well as preparation method and application thereof
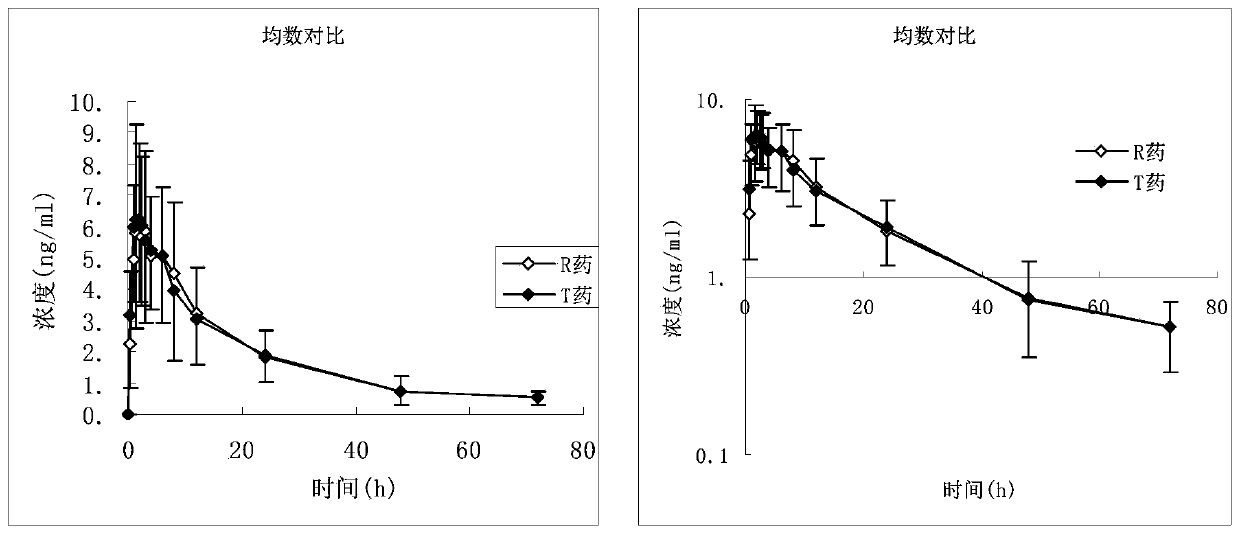
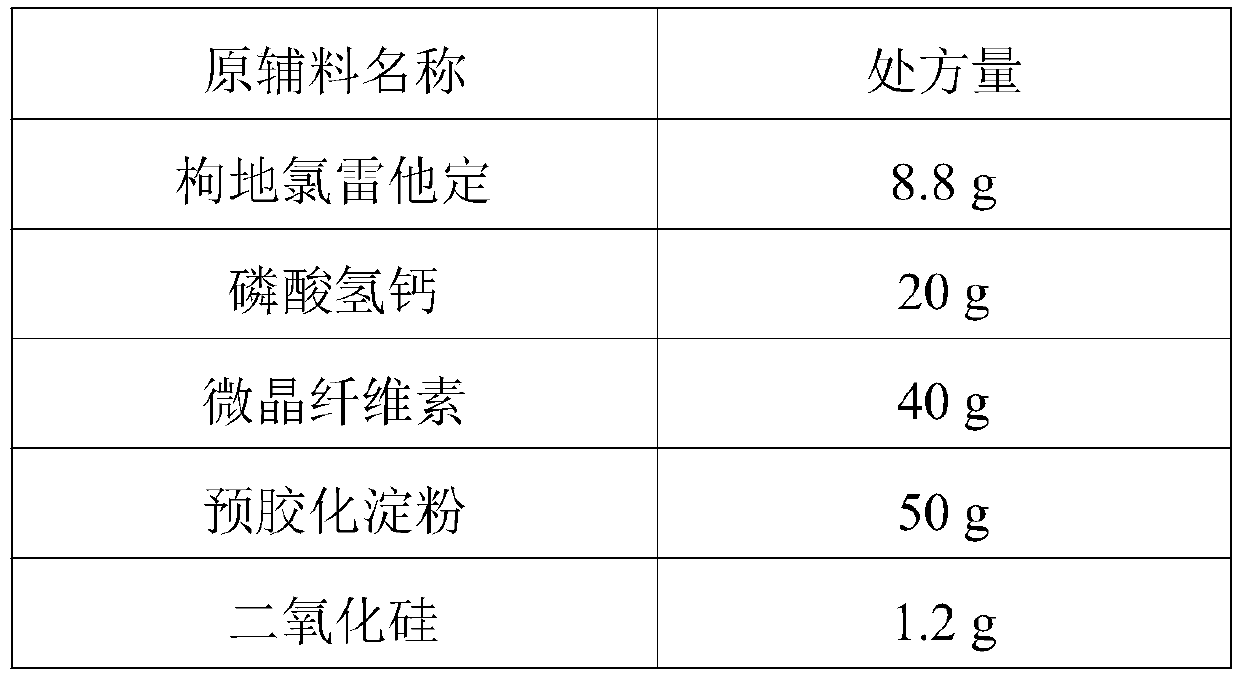
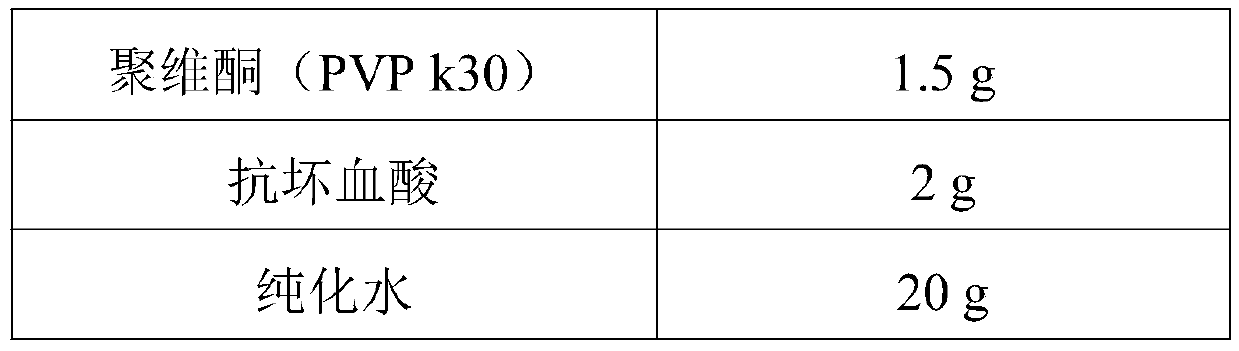
ActiveCN111419820AReasonable formulaSimple production processOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical SubstancesNuclear chemistry
The invention provides desloratadine citrate disodium capsules as well as a preparation method and application thereof. The capsules are composed of desloratadine citrate disodium particles and hollowcapsules, and the desloratadine citrate disodium particles contain desloratadine citrate disodium, ascorbic acid, a filler, a lubricant and an adhesive in a weight percentage ratio of (5-12):(1-3):(80-150):(0.5-2):(0.5-3). The invention also provides a preparation method of the desloratadine citrate disodium capsules and application of the capsules in preparation of medicines for treating rhinitis and urticaria. The desloratadine citrate disodium capsules disclosed by the invention are reasonable in formula and simple and convenient in production process; and by adding the ascorbic acid component, the dissolution rate and the stability of the desloratadine citrate disodium capsules are remarkably improved.
Owner:HEFEI IND PHARMA INST CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com