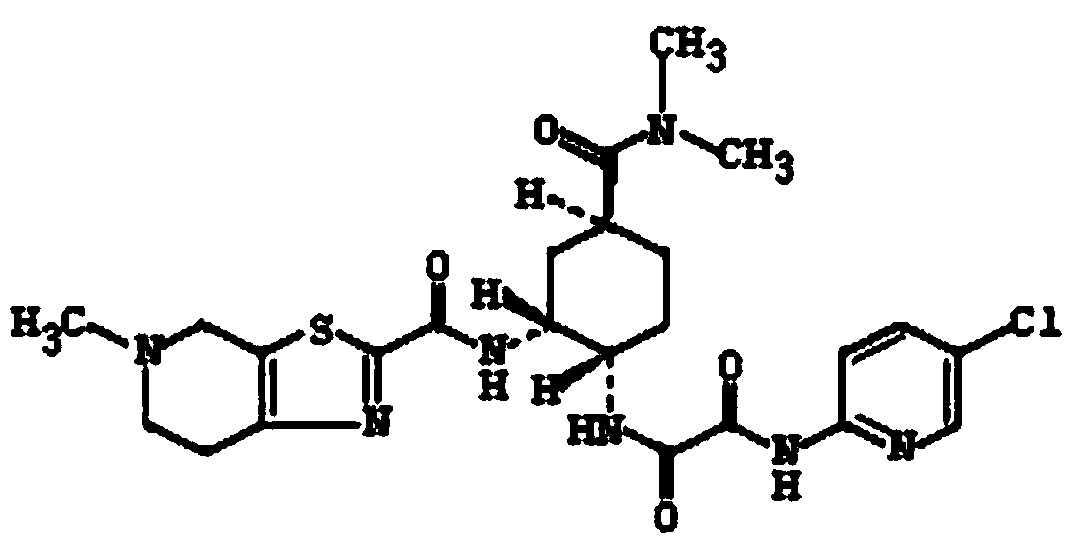
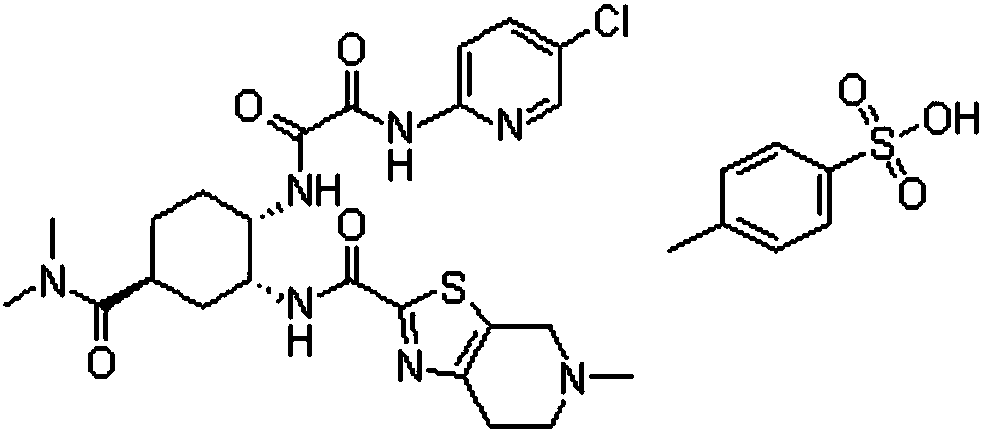
A kind of edoxaban tosylate tablet and preparation method thereof
A technology of edoxaban tosylate and tablet, applied in the field of medicine, can solve the problems of unfavorable clinical treatment, reduced bioavailability, poor dissolution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, preparation edoxaban tosylate sheet
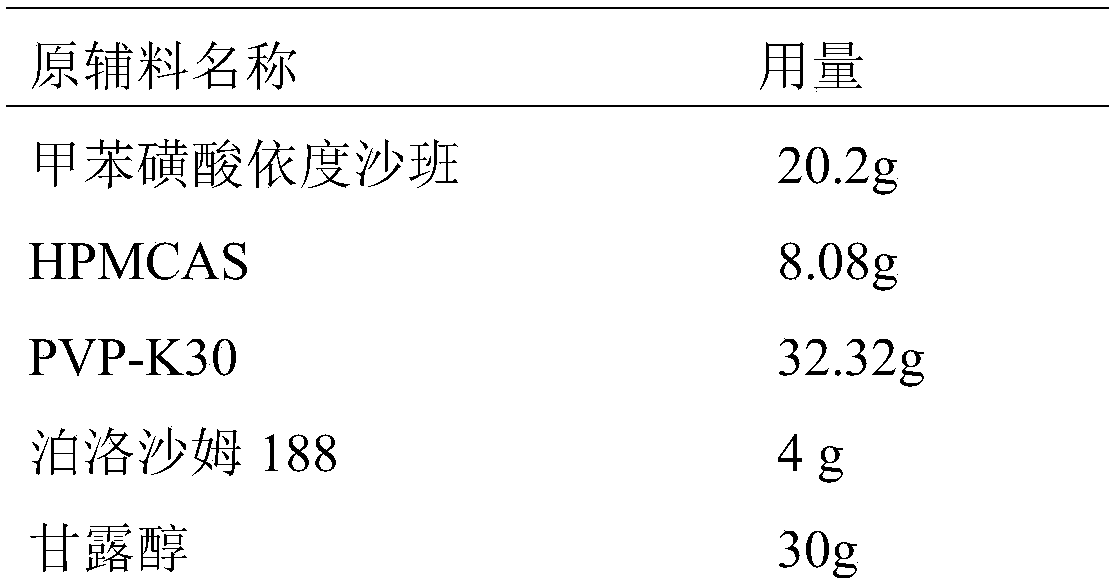
[0040] The present embodiment provides the prescription and preparation method for preparing 1000 Edoxaban Tosylate Tablets (the specification is 15 mg / tablet, calculated as Edoxaban), as follows:
[0041] Prescription composition:
[0042]
[0043]
[0044] Crushing the raw material edoxaban toluenesulfonate (D90≤25 μm) through a 100-mesh sieve, mannitol and pregelatinized starch as auxiliary materials, respectively, through a 80-mesh sieve, and magnesium stearate through a 100-mesh sieve;
[0045] The preparation method is as follows:
[0046] 1) Weigh the prescription amount of edoxaban tosylate, hydrophilic polymer carrier (HPMCAS, PVP-K30), surfactant (poloxamer 188) and dissolve in 2.5L solvent (dimethyl sulfoxide In the mixed solvent that is 1:4 with water volume ratio), adopt ultrasonic-assisted dissolving to obtain homogeneous solution; Then remove described solvent with spray drying, obtain edoxaban tos...
Embodiment 2
[0049] Embodiment 2, preparation Edoxaban tosylate sheet
[0050] The present embodiment provides the prescription and preparation method for preparing 1000 Edoxaban Tosylate Tablets (the specification is 30 mg / tablet, calculated as Edoxaban), as follows:
[0051] Prescription composition:
[0052]
[0053]
[0054] The preparation method is basically the same as in Example 1.
Embodiment 3
[0055] Embodiment 3, preparation Edoxaban tosylate sheet
[0056] The present embodiment provides the prescription and preparation method for preparing 1000 Edoxaban Tosylate Tablets (the specification is 15 mg / tablet, calculated as Edoxaban), as follows:
[0057] Prescription composition:
[0058]
[0059] The preparation method is basically the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com