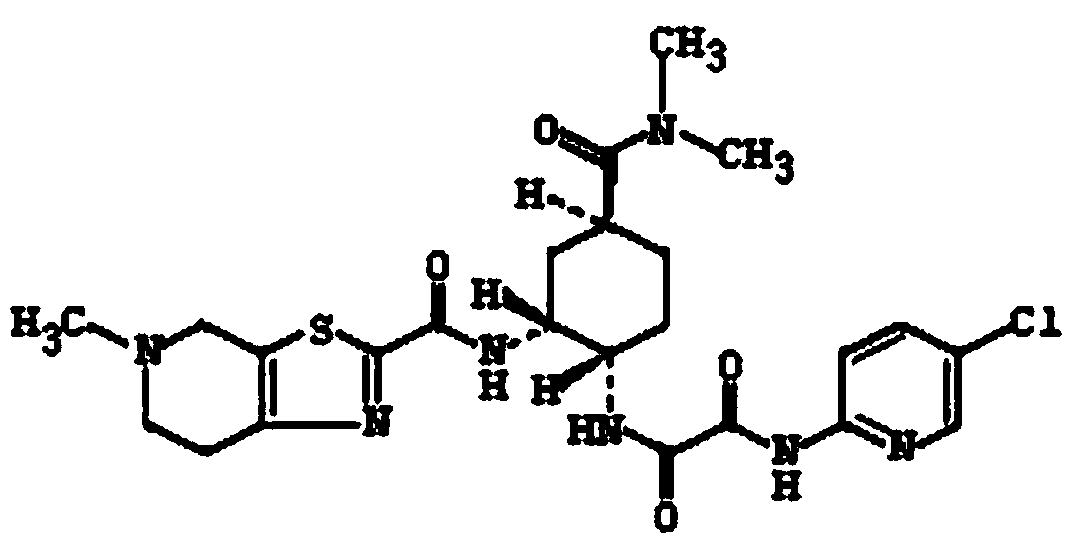
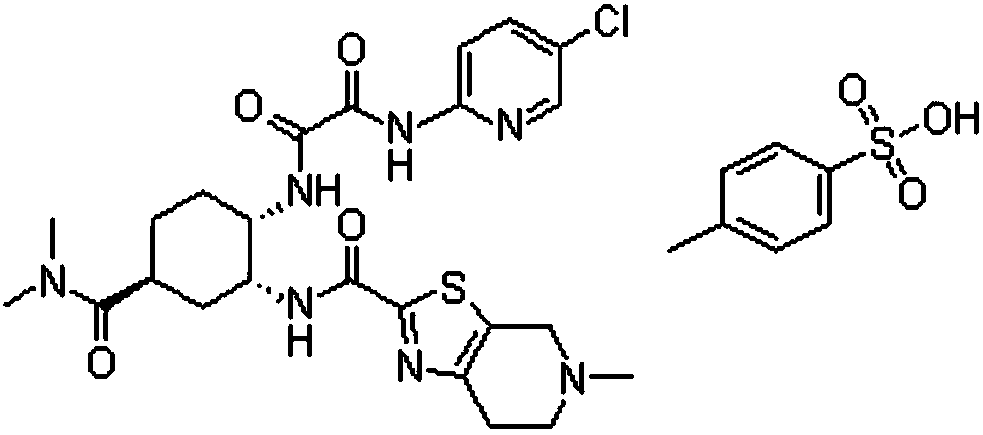
Toluenesulfonic acid edoxaban tablet and preparation method thereof
A technology of edoxaban tosylate and tablets, which is applied in the field of medicine and can solve problems such as poor dissolution, affecting drug absorption, and unfavorable clinical treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
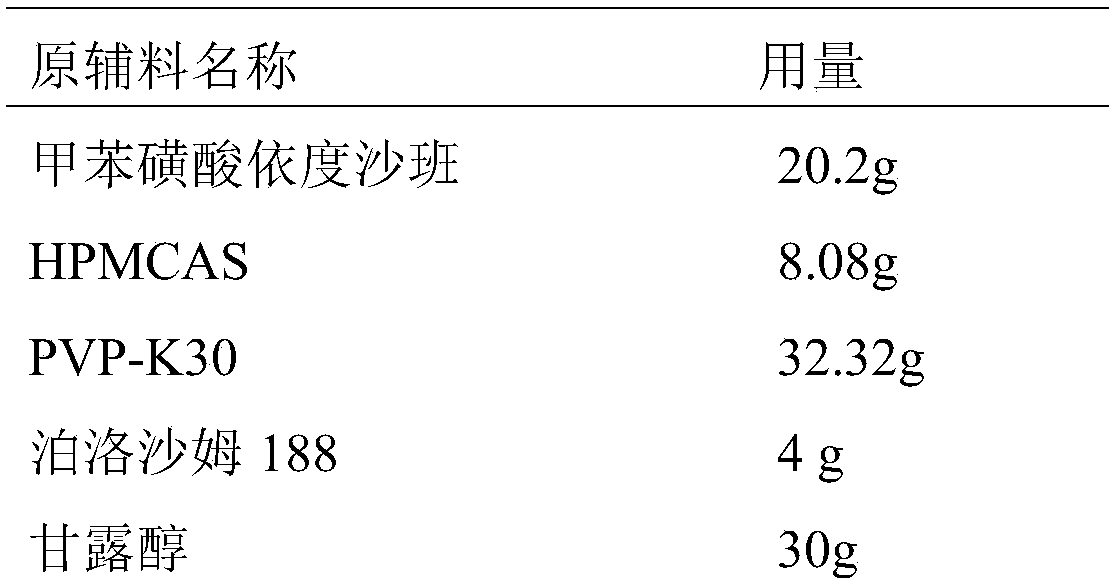
[0039] Embodiment 1, preparation edoxaban tosylate sheet
[0040] The present embodiment provides the prescription and preparation method for preparing 1000 Edoxaban Tosylate Tablets (the specification is 15 mg / tablet, calculated as Edoxaban), as follows:
[0041] Prescription composition:
[0042]
[0043]
[0044] Crushing the raw material edoxaban toluenesulfonate (D90≤25 μm) through a 100-mesh sieve, mannitol and pregelatinized starch as auxiliary materials, respectively, through a 80-mesh sieve, and magnesium stearate through a 100-mesh sieve;
[0045] The preparation method is as follows:
[0046] 1) Weigh the prescription amount of edoxaban tosylate, hydrophilic polymer carrier (HPMCAS, PVP-K30), surfactant (poloxamer 188) and dissolve in 2.5L solvent (dimethyl sulfoxide In the mixed solvent that is 1:4 with water volume ratio), adopt ultrasonic-assisted dissolving to obtain homogeneous solution; Then remove described solvent with spray drying, obtain edoxaban tos...
Embodiment 2
[0049] Embodiment 2, preparation Edoxaban tosylate sheet
[0050] The present embodiment provides the prescription and preparation method for preparing 1000 Edoxaban Tosylate Tablets (the specification is 30 mg / tablet, calculated as Edoxaban), as follows:
[0051] Prescription composition:
[0052]
[0053]
[0054] The preparation method is basically the same as in Example 1.
Embodiment 3
[0055] Embodiment 3, preparation Edoxaban tosylate sheet
[0056] The present embodiment provides the prescription and preparation method for preparing 1000 Edoxaban Tosylate Tablets (the specification is 15 mg / tablet, calculated as Edoxaban), as follows:
[0057] Prescription composition:
[0058]
[0059] The preparation method is basically the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com