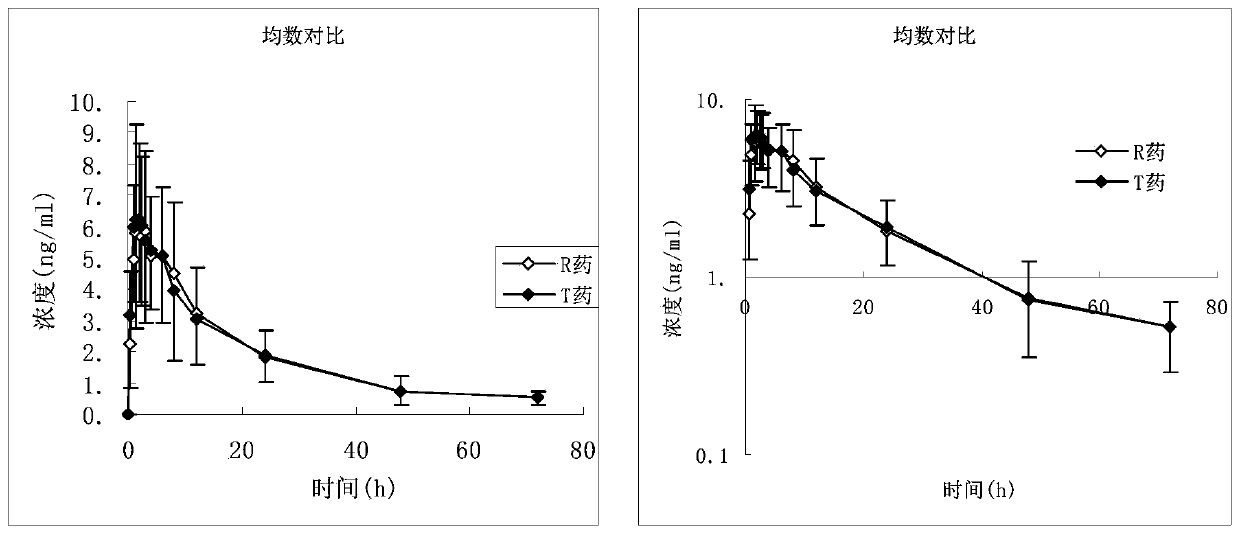
Desloratadine citrate disodium capsules as well as preparation method and application thereof
A technology of desloratadine and loratadine, which is applied in the field of pharmaceutical preparations, can solve the problems that the substance content of desloratadine cannot be solved well, so as to improve the dissolution rate and stability, improve the dissolution behavior, Effect of inhibiting the growth of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029]
[0030] The preparation method is:
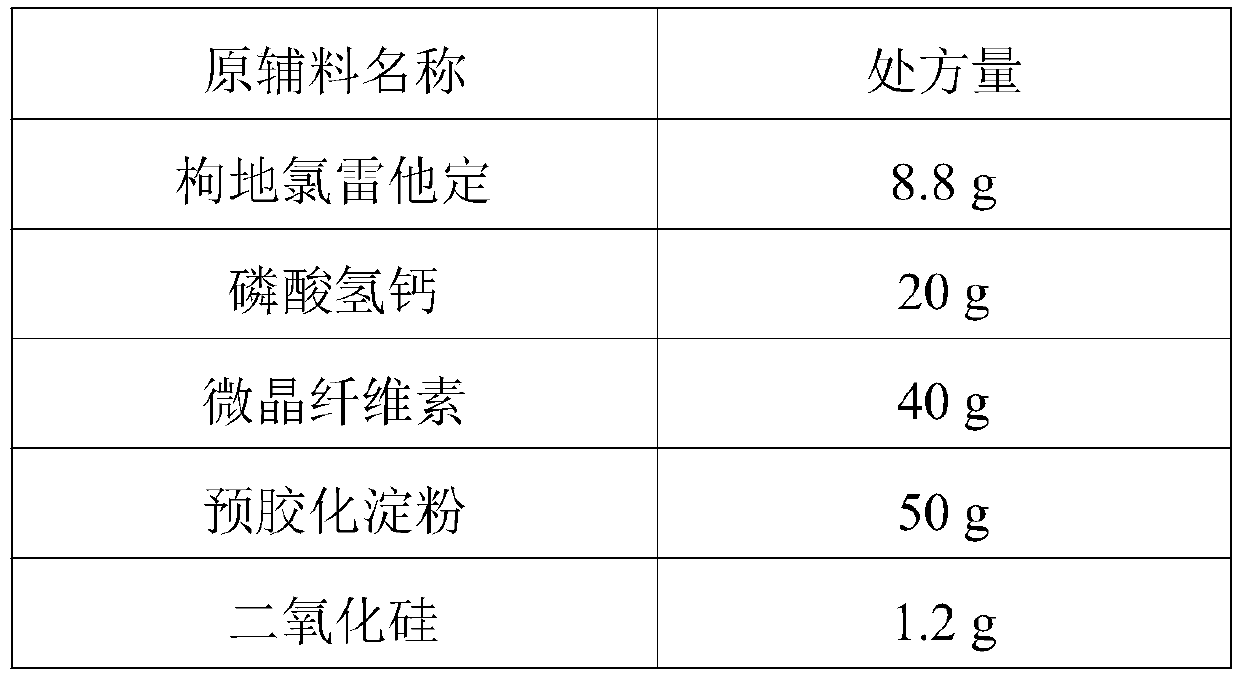
[0031] (1) Desloratadine citrate, ascorbic acid, calcium hydrogen phosphate, microcrystalline cellulose and pregelatinized starch are taken by weighing the prescription amount, sieved and premixed with 60 meshes;
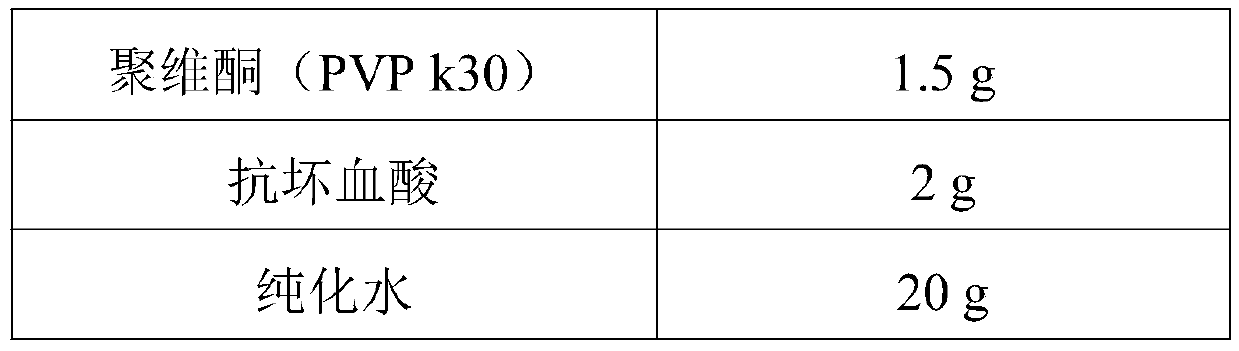
[0032] (2) Weigh the PVP k30 of the prescribed amount, add the prescribed amount of purified water to prepare a binder solution, and add it to the mixture prepared in step (1), granulate with a 30-mesh sieve, and dry in a fluidized bed until the moisture content is ≤5.0%. After sizing through a 24-mesh sieve, add silicon dioxide for total mixing to obtain desloratadine citrate granules;
[0033] (3) Fill the desloratadine citrate granules into gelatin hollow capsules (No. 3) to prepare 1000 desloratadine citrate capsules.
Embodiment 2
[0035] Name of raw material Prescription amount desloratadine citrate 8.8g Calcium hydrogen phosphate 45g microcrystalline cellulose 65g silica 1.2g Povidone (PVPk30) 1.5g ascorbic acid 1g purified water 20g
[0036] The preparation method is:
[0037] (1) take desloratadine citrate, ascorbic acid, calcium hydrogen phosphate and microcrystalline cellulose of prescription quantity, 60 mesh sieve, premix;
[0038] (2) Weigh the PVP k30 of the prescribed amount, add the prescribed amount of purified water to prepare a binder solution, and add it to the mixture prepared in step (1), granulate with a 30-mesh sieve, and dry in a fluidized bed until the moisture content is ≤5.0%. After sizing through a 24-mesh sieve, add silicon dioxide for total mixing to obtain desloratadine citrate granules;
[0039] (3) Fill the desloratadine citrate granules into gelatin hollow capsules (No. 4) to prepare 1000 desloratadine citrate capsu...
Embodiment 3
[0041] Name of raw material Prescription amount desloratadine citrate 8.8g Calcium hydrogen phosphate 40g pregelatinized starch 70g silica 1.2g Povidone (PVPk30) 1.5g ascorbic acid 3g purified water 20g
[0042] The preparation method is:
[0043] (1) take by weighing desloratadine citrate, ascorbic acid, calcium hydrogen phosphate and pregelatinized starch of prescription quantity, 60 mesh sieve, premix;
[0044] (2) Weigh the PVP k30 of the prescribed amount, add the prescribed amount of purified water to prepare a binder solution, and add it to the mixture prepared in step (1), granulate with a 30-mesh sieve, and dry in a fluidized bed until the moisture content is ≤5.0%. After sizing through a 24-mesh sieve, add silicon dioxide for total mixing to obtain desloratadine citrate granules;
[0045] (3) Fill the desloratadine citrate granules into gelatin hollow capsules (No. 4) to prepare 1000 desloratadine citrate cap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com