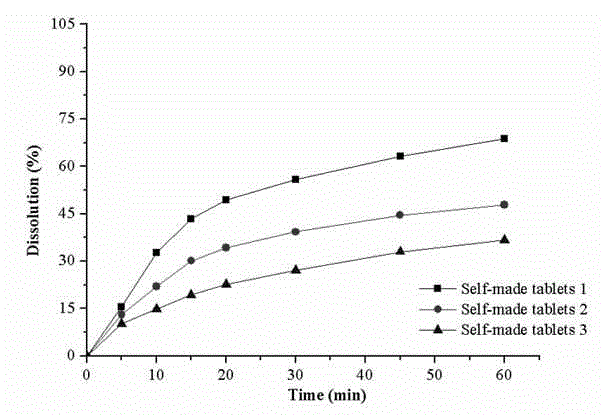
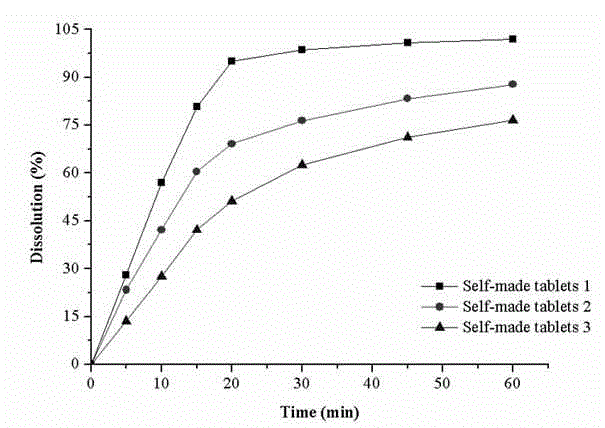
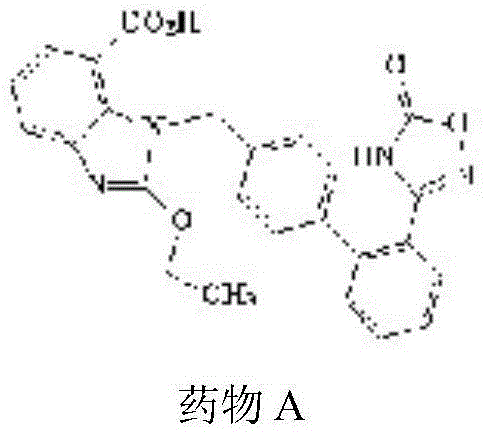
Preparation and application of drug powder
A drug and powder technology, which is applied in the field of pharmaceutical preparations, can solve the problems of easy moisture absorption and aging dissolution of solid dispersions, difficulty in avoiding the aggregation of hydrophobic drug A, and the inability to reduce the particle size of drug A, so as to achieve good dissolution behavior and improve Isolation effect, effect of improving grinding efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Prepare drug A suspension with different hydrophilic suspending agents
[0030] Drug A suspension prescription:
[0031] Drug A 20g
[0032] Hydrophilic suspension 4g
[0033] Distilled water 200ml
[0034] Grinding with a sand mill for a suitable time to obtain a suspension
[0035] Preparation process: 4g of hydroxypropylcellulose, hydroxypropylmethylcellulose, polyvinylpyrrolidone, and polyvinyl alcohol are respectively dissolved in 200ml of distilled water, and 20g of drug A is added under stirring to fully suspend. Use the ESW-750 laboratory sand mill (Shanghai Yile Electromechanical Co., Ltd.) to grind the suspension for a suitable time, and use the LS-230 particle size analyzer (Beckman Company) to measure the distribution of drug particles, as shown in Table 1.
[0036] Table 1 The influence of different hydrophilic suspending agents on the drug particle size after grinding
[0037]
[0038] The results showed that using hydroxypropyl cel...
Embodiment 2
[0039] Embodiment 2: Different concentrations of hydroxypropyl cellulose are used as suspending agent to prepare drug A suspension
[0040] Drug A suspension prescription:
[0041] Drug A 20g
[0042] Proper amount of hydroxypropyl cellulose
[0043] Distilled water 200ml
[0044] Grinding with a sand mill for a suitable time to obtain a suspension
[0045] Preparation process: Dissolve 1, 2, 4, 6, and 10 g of hydroxypropyl fiber in 200 ml of distilled water, respectively, and add 20 g of drug A under stirring to fully suspend it. Use the ESW-750 laboratory sand mill (Shanghai Yile Electromechanical Co., Ltd.) to grind the suspension for a suitable time, and use the LS-230 particle size analyzer (Beckman Company) to measure the distribution of drug particles, as shown in Table 2.
[0046] Table 2 Effect of different concentrations of hydroxypropyl cellulose on drug particle size after grinding
[0047]
[0048] The results showed that different hydroxypropyl cellulose ...
Embodiment 3
[0049] The impact of the mass ratio of embodiment 3 medicine and hydroxypropyl cellulose on grinding effect
[0050] Drug A suspension prescription:
[0051] Drug A appropriate amount
[0052] Hydroxypropyl Cellulose 4g
[0053] Distilled water 200ml
[0054] Grinding with a sand mill for a suitable time to obtain a suspension
[0055] After dissolving 4g of hydroxypropyl fiber in 200ml of distilled water, add 4, 10, 20, 40, 80, 120, and 160g of drug A under stirring to fully suspend it. Use the ESW-750 laboratory sand mill (Shanghai Yile Electromechanical Co., Ltd.) to grind the suspension for a suitable time, and use the LS-230 particle size analyzer (Beckman Company) to measure the distribution of drug particles, as shown in Table 3.
[0056] Table 3 Effect of co-grinding hydroxypropyl cellulose with different proportions of drugs on drug particle size
[0057]
[0058] The results showed that with the increase of the drug ratio, the effect of grinding on reducing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com