Patents
Literature
79results about How to "Improve in vitro dissolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
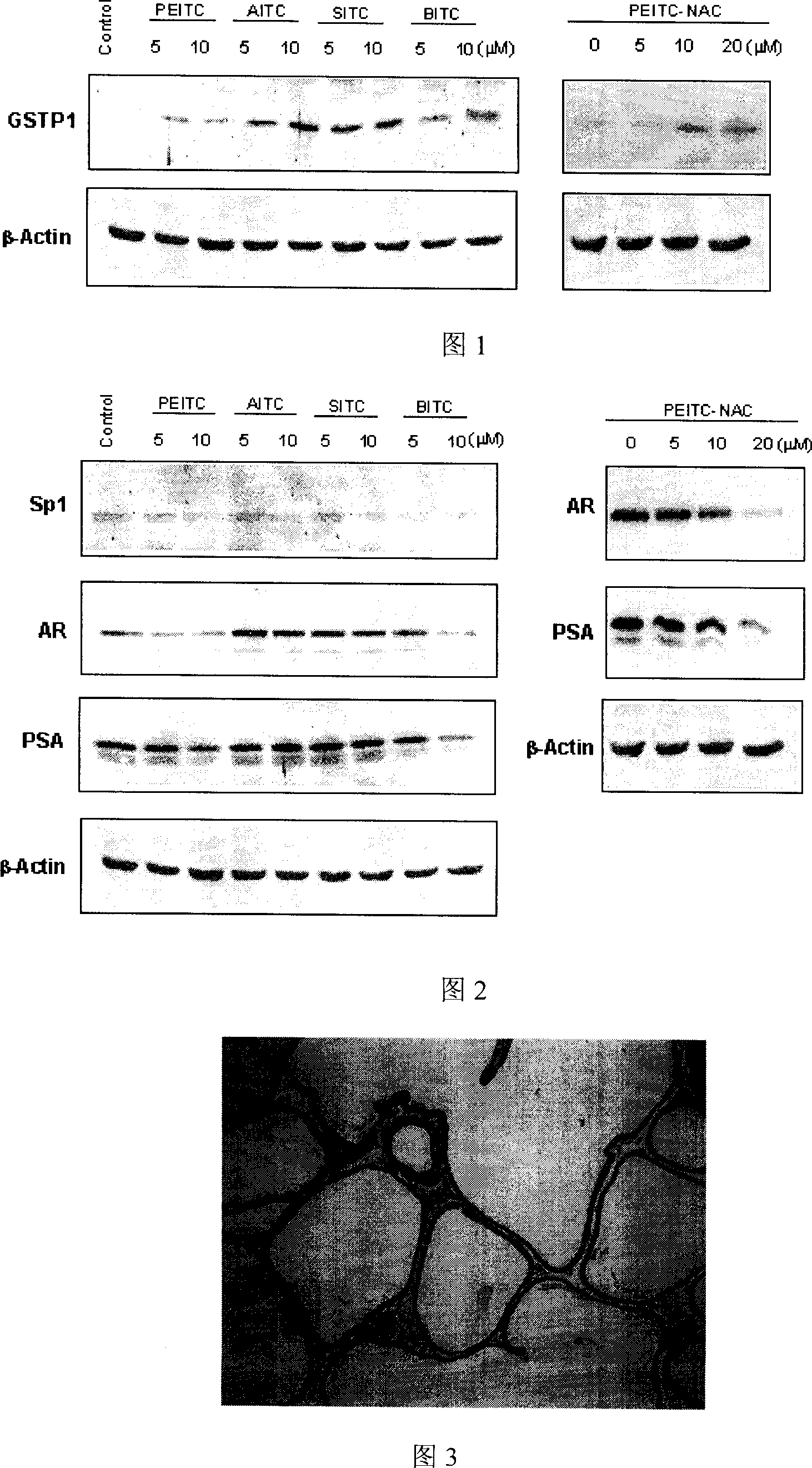
Application of compounds in isorhodanic ester classes for treating diseases of prostate and skin cancer
ActiveCN101091705AIncreased ability to remove harmful substancesImprove in vitro dissolutionUrinary disorderEster active ingredientsDiseaseProstate cancer cell
The present invention relates to a method capable of using natural and artificial synthetic isosulfocyanate compound or its derivative to prevent and cure prostatic diseases and skin carcinoma. The internal tests show that various isosulfocyanate compounds or their derivatives can induce prostatic cell II phase drug metabolic detoxication enzyme-glutathione transferase so as to can inhibit the hyperplasia of prostate and inflammation, and can prevent and cure prostatic carcinoma and skin carcinoma.
Owner:JC (WUXI) CO INC
Iguratimod oral double-layer sustained-release preparation
InactiveCN101095671AAccelerate time to peak blood concentrationImprove in vitro dissolutionOrganic active ingredientsAntipyreticSide effectEffective action
The invention relates to oral double iguratimod controlled release formulation, which comprises fast release layer and slow release layer that are composed of 8-30% micronizing iguratimod crystal powder and medical findings, and the granule size of iguratimod crystal powder is 1-10 um. The effective component in fast release layer is released in short time and reaches to effective blood chemical concentration for effective action; the iguratimod in sloe release layer is released gradually and maintains effective blood medical concentration for continuous effective action. The invention overcomes shortcomings of short effective action time and a little high toxic effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Furazolidone tablet preparation method
ActiveCN103271887ASimple methodEasy to operateAntibacterial agentsOrganic active ingredientsAlcoholMedicine
Owner:YUNNAN PHYTOPHARML
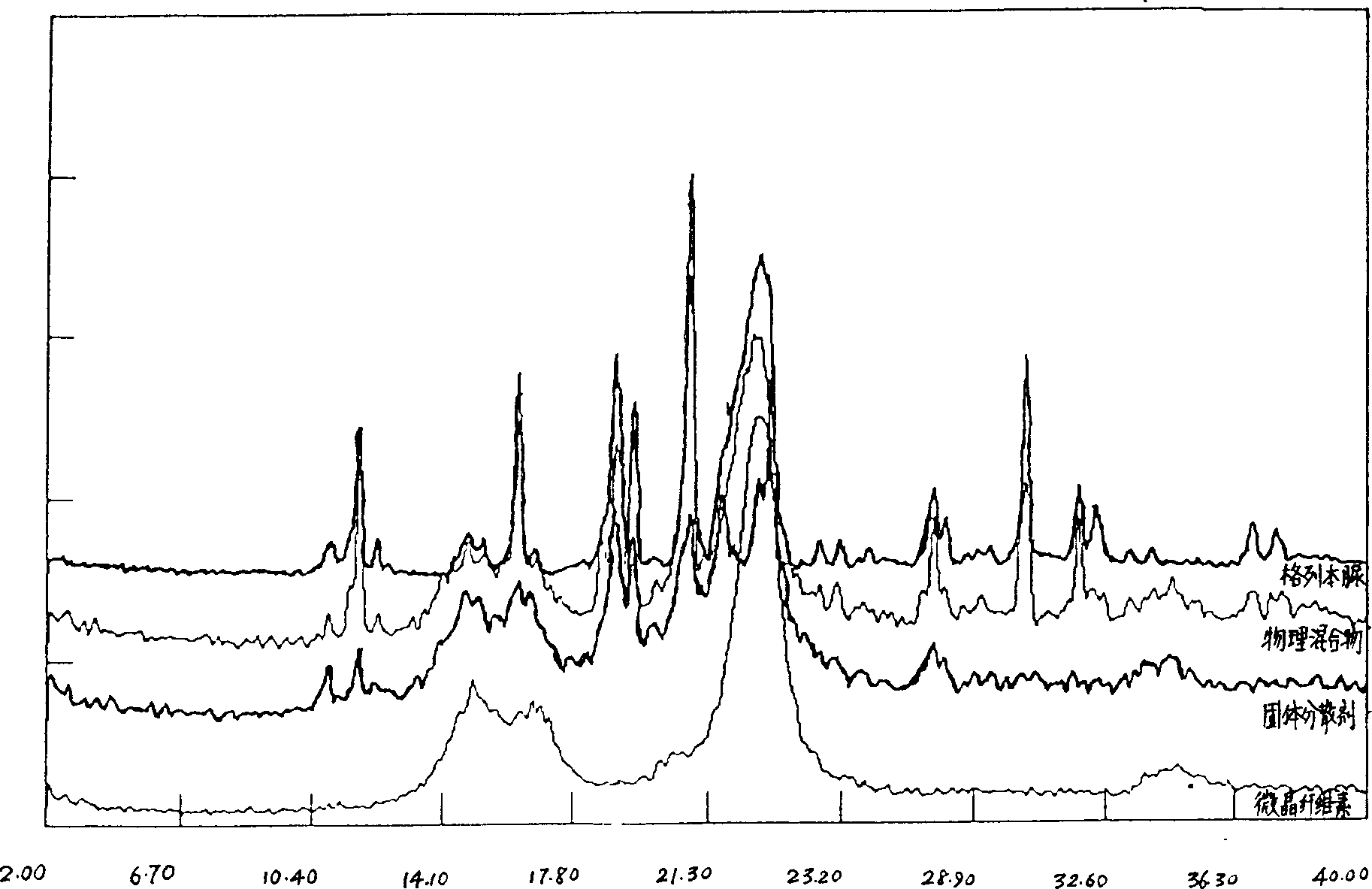
Solid dispersion and preoral combination of glibenclamide and preparation method
ActiveCN1660057AInhibition of dissolution rateDoes not change the chemical structureMetabolism disorderSulfonylurea active ingredientsSolubilityDispersed media
A dispersed solid or orally taken composition of gliben clamide clamide is prepared from glibenclamide and dispersing medium proportionally through mixing, grinding and fusing or spray drying.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
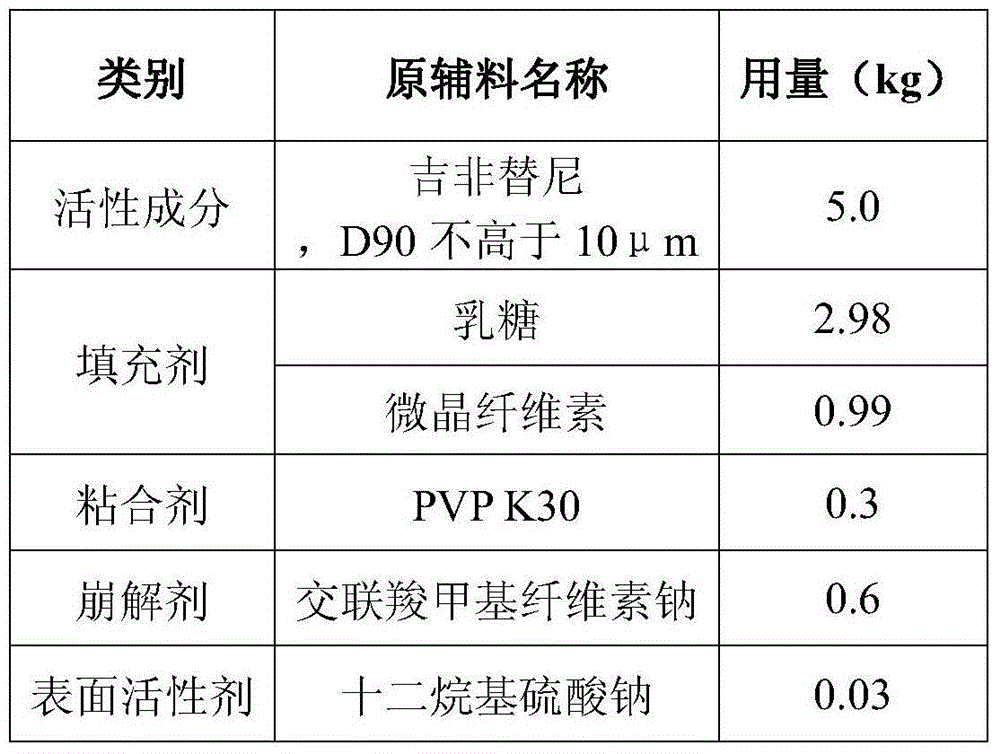
Gefitinib tablet preparation method
InactiveCN104800175AImprove in vitro dissolutionImprove stabilityOrganic active ingredientsPill deliveryMedicineLactose
The invention relates to the field of medicines, in particular to a gefitinib tablet preparation method. The gefitinib tablet mainly comprises a main medicine and auxiliary materials. The gefitinib tablet preparation method is characterized in that the gefitinib particle diameter D90 is not more than 10 [mu]m, the proportion of lactose to microcrystalline cellulose is 1: (3-7), uniform mixing is performed according to the formula dosage, and then pelletizing, drying, granulating and tabletting are carried out. The prepared gefitinib tablet is stable in property, reliable in quality and high in bioavailability.
Owner:珠海润都制药股份有限公司
Montelukast sodium chewable tablet, preparation method and determination method of dissolution rate
InactiveCN104146975AImprove in vitro dissolutionGuaranteed stabilityAntipyreticComponent separationMANNITOL/SORBITOLDissolution
The invention relates to the field of medicinal preparations and particularly relates to a montelukast sodium chewable tablet, apreparation method and a determination method of a dissolution rate. Raw materials of the montelukast sodium chewable tablet comprise montelukast sodium, mannitol, microcrystalline cellulose, hydroxy propyl cellulose, crosslinking sodium carboxymethylcellulose, aspartame, ferric oxide, magnesium stearate and cherry essence. The hydroxy propyl cellulose is prepared into a solution with a mass percentage content being 6-8% through water being a solvent and a disintegrating agent is prepared through a wet process of an internal addition method. The montelukast sodium chewable tablet is significantly increased in disintegrating speed and in-vitro dissolution rate. By means of a dissolution curve detection method, quality differences among products in each batch can be effectively distinguished. Differences between products in each batch can be reduced better and a quality risk of the products can be controlled in a controllable range.
Owner:BENGBU BBCA MEDICINE SCI DEV
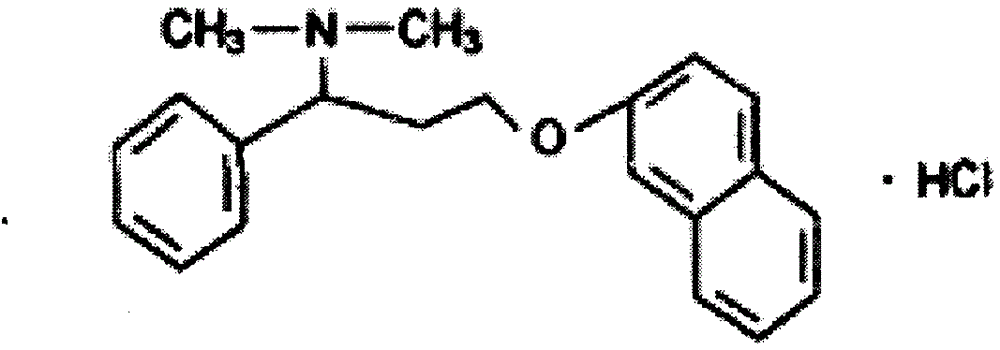
Hydrochloric acid dapoxetine tablet
ActiveCN105232503AReduce the impactGentle curveOrganic active ingredientsNervous disorderAcetic acidOrganic solvent
The invention relates to a hydrochloric acid dapoxetine tablet and belongs to the field of medicine production new technologies, in particular to the hydrochloric acid dapoxetine tablet and a preparation method thereof. Hydrochloric acid dapoxetine serves as the main constituent, and the tablet is obtained through direct tabletting of hydrochloric acid dapoxetine solid dispersion particles and auxiliary materials; a preparation method of the hydrochloric acid dapoxetine solid dispersion particles comprises the steps of mixing hydrochloric acid dapoxetine with organic solvent for forming a clear solution, and then adding the clear solution to dextrin for conducting pelleting. The weight ratio of hydrochloric acid dapoxetine and dextrin is 1 to 2-15, and the organic solvent is one or more out of ethanol, acetic acid, ethyl acetate and acetone. By means of the hydrochloric acid dapoxetine tablet, the influence of crystal form difference on preparations is eliminated, and the obtained hydrochloric acid dapoxetine tablet is good in in-vitro dissolution, safe and stable.
Owner:NANJING ZENKOM PHARMA
Pharmaceutical composition for improving in-vitro dissolution and liquidity of spironolactone
ActiveCN105832680AImprove in vitro dissolutionImprove liquidityOrganic active ingredientsPill deliveryOrganic solventMass ratio
The invention belongs to the field of pharmaceutical preparations, and relates to a pharmaceutical composition for improving in-vitro dissolution and liquidity of spironolactone and a preparation method of the pharmaceutical composition. The pharmaceutical composition is mainly characterized by being prepared from an indissolvable drug spironolactone and a carrier material, wherein the mass ratio of the drug to the carrier is 1:3-1:10, the pharmaceutical composition prepared through a hot-melt extrusion technique is solid dispersion, the spironolactone is dispersed into the carrier in a molecular or amorphous mode, and therefore the in-vitro dissolution of the spironolactone is significantly improved. After the spironolactone is smashed, the liquidity of the spironolactone is significantly improved, and the smashed spironolactone can be directly filled into capsules or directly subpackaged by serving as powder and granules or used by being mixed with other pharmaceutical auxiliary materials to prepare tablets. Compared with traditional methods such as the solvent method and the solvent-melt method, the adopted hot-melt extrusion technique has the advantages of being capable of not using an organic solvent, safe, free of pollution, stable in technology, capable of achieving continuous operation, easily achieving industrialized enlarged production and improving the liquidity without needing to add a flow aid and the like.
Owner:SHENYANG PHARMA UNIVERSITY
Lipoic acid capsules as well as preparation process and application thereof
ActiveCN102579395AImprove in vitro dissolutionReduce dosageOrganic active ingredientsAntinoxious agentsDissolutionCarrier material
The invention relates to lipoic acid capsules. The capsules comprise solid dispersoid. The solid dispersoid consists of lipoic acid and a carrier material. The carrier material is selected from poloxamer. According to the invention, the dissolution in vitro of a lipoic acid preparation is improved.
Owner:JIANGSU WANHE PHARMA
Cilnidipine nano-suspension and preparation method thereof
PendingCN110711176AImprove physical stabilityIncrease dissolution rateOrganic active ingredientsSolution deliveryCilnidipineLiquid state
The present invention relates to a cilnidipine nano-suspension and a preparation method thereof. The cilnidipine nano-suspension is obtained by a nanometer treatment of cilnidipine and a stabilizer aqueous solution, wherein the stabilizer is a space stabilizer, a charge stabilizer or a combination of the space stabilizer and the charge stabilizer. The prepared cilnidipine nano-suspension has characteristics of high dissolution and high bioavailability, can be used as a preparation finished product or as a preparation intermediate to prepare a solid or liquid state cilnidipine preparation, andcan ensure medicine absorption and medicine effect stability. Besides, the processing technology is feasible, stable and reliable, and provides an easily industrialized technical means and preparationintermediate preparation technology for development of the cinidipine preparation, and thus the manufactured cilnidipine preparation has good oral absorption and application prospects.
Owner:NINGXIA MEDICAL UNIV
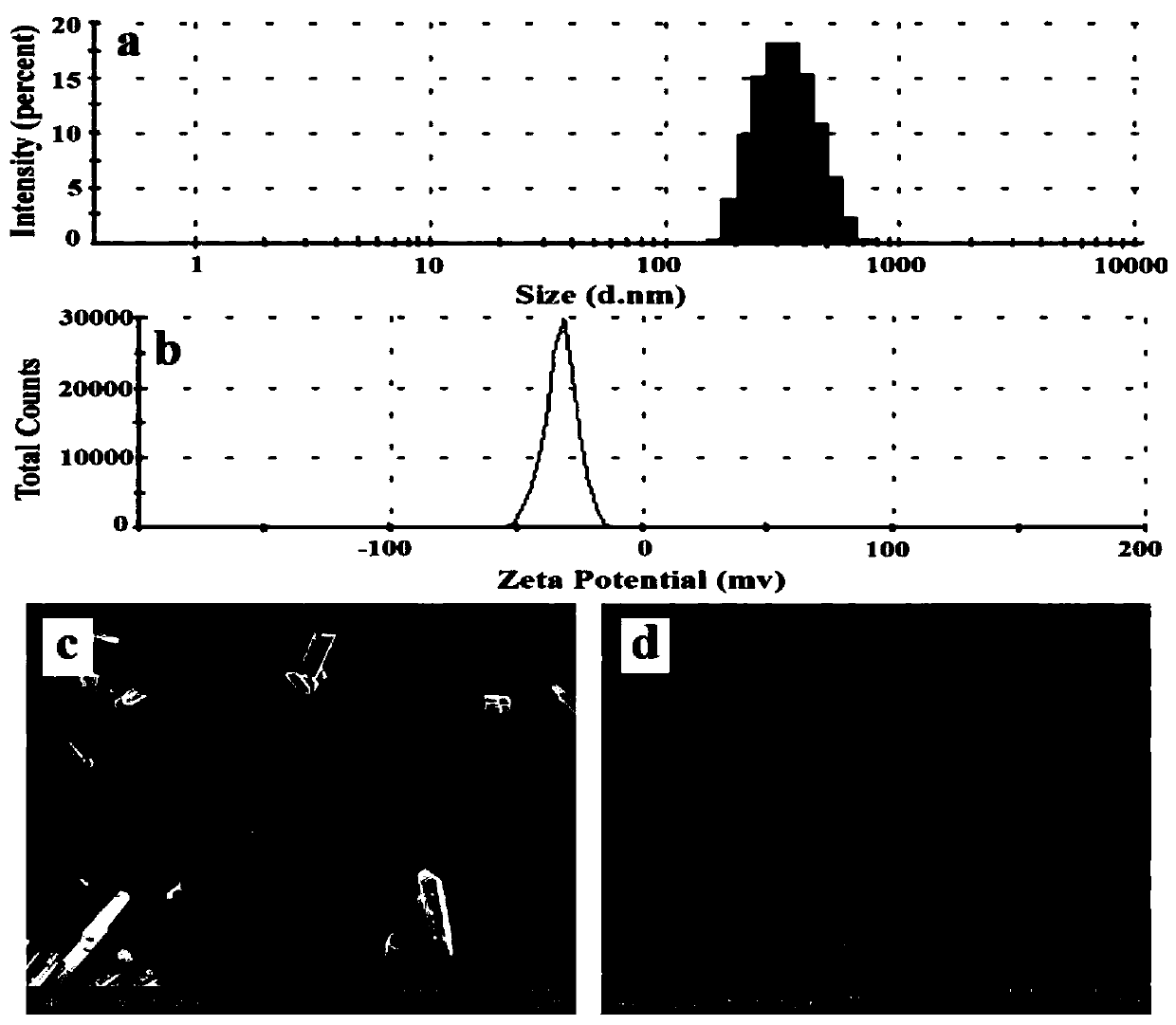
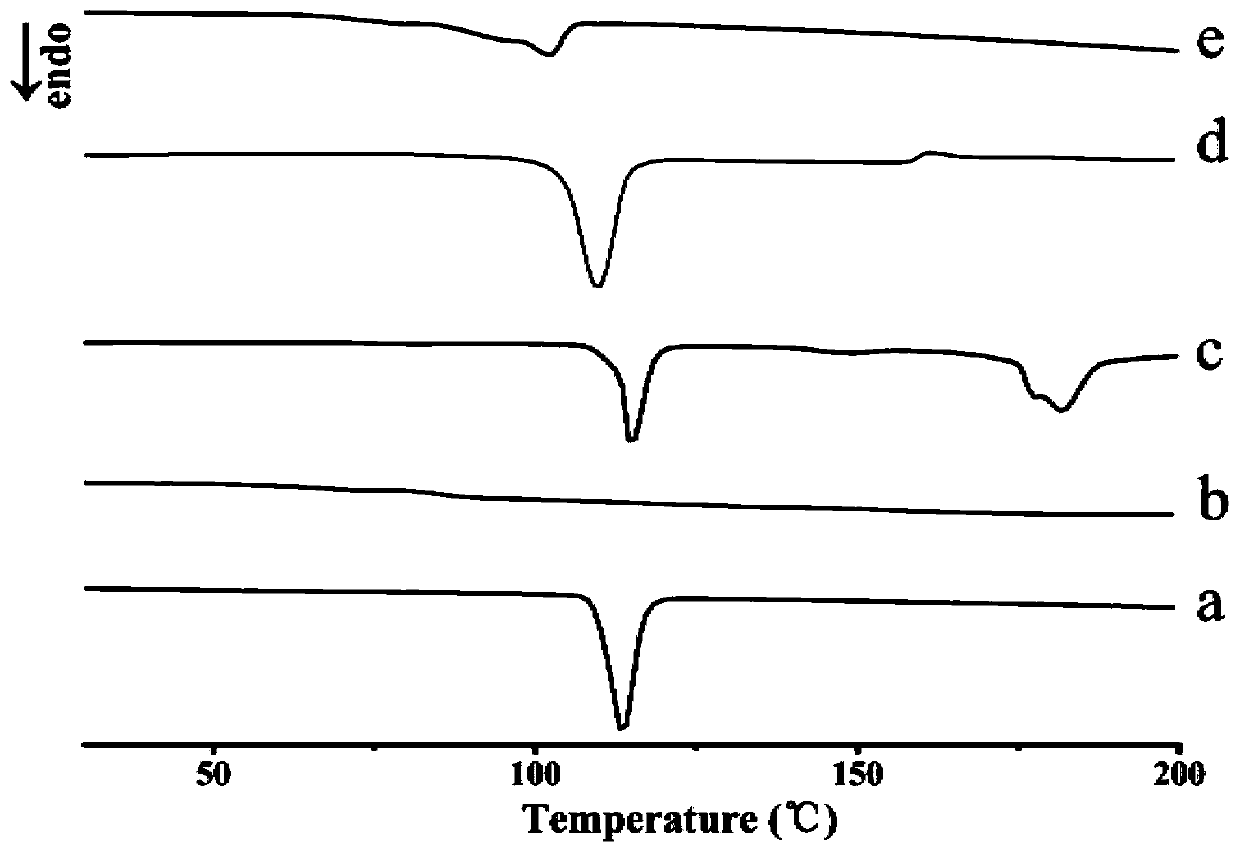
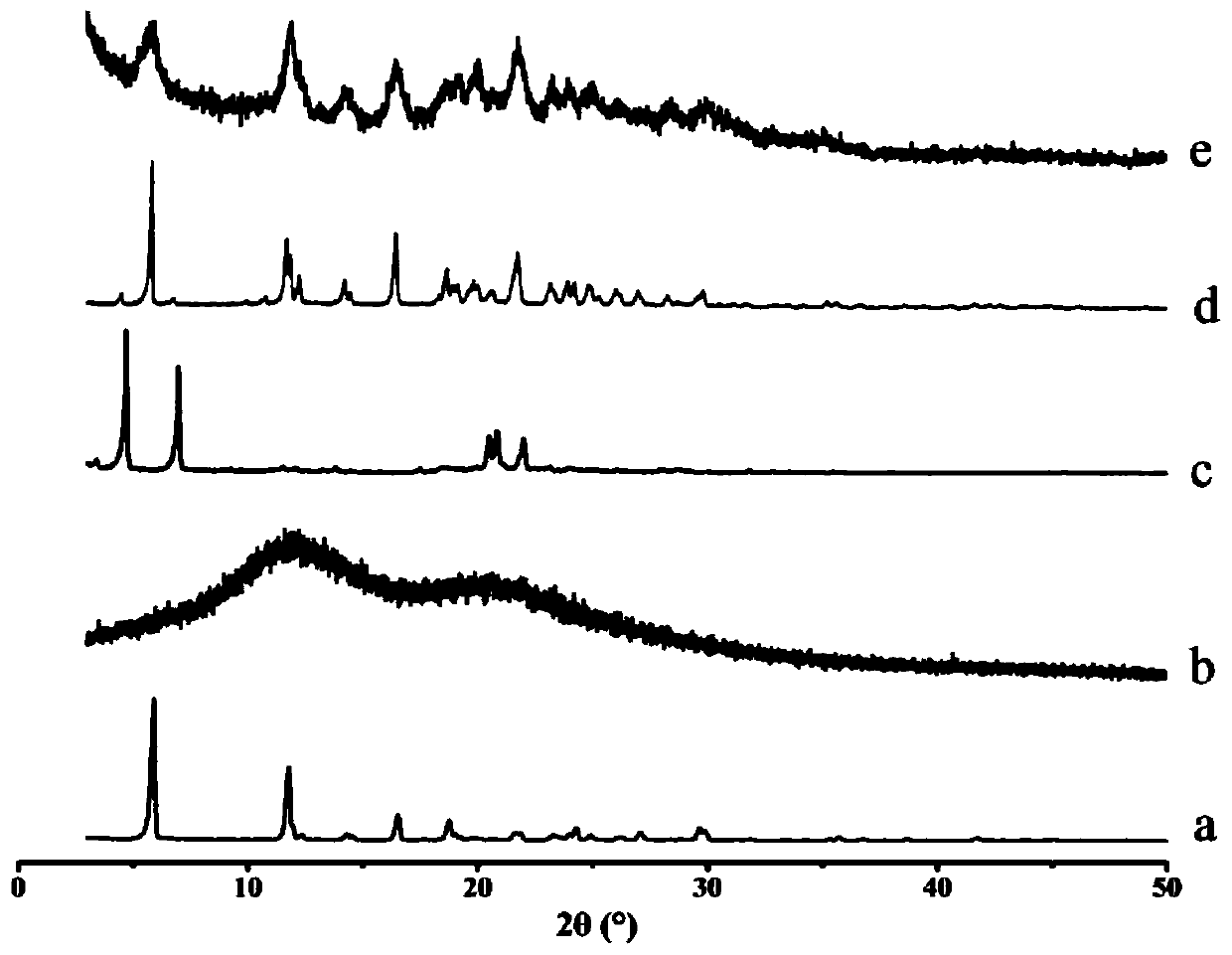
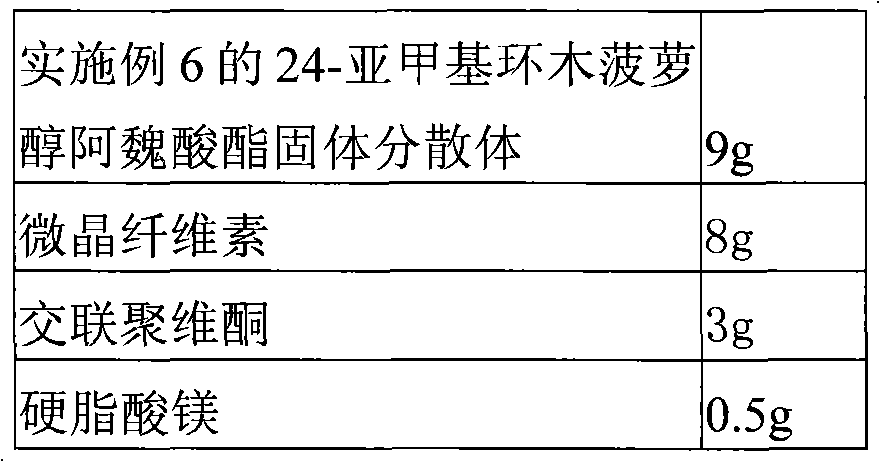
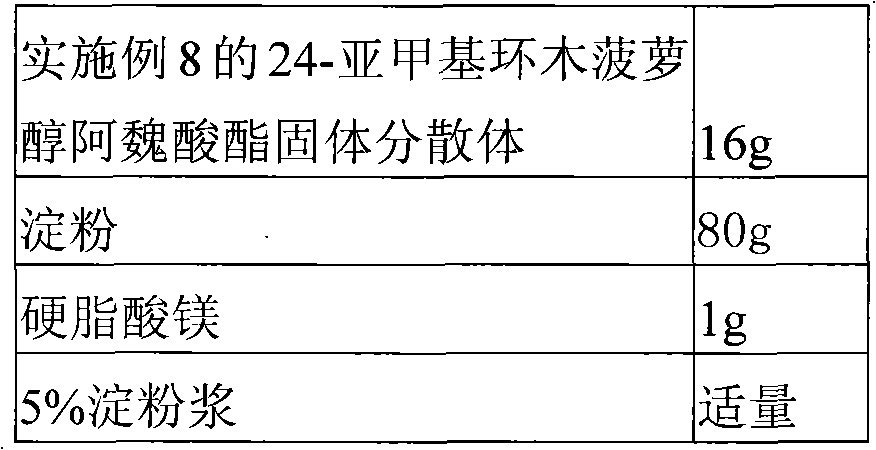
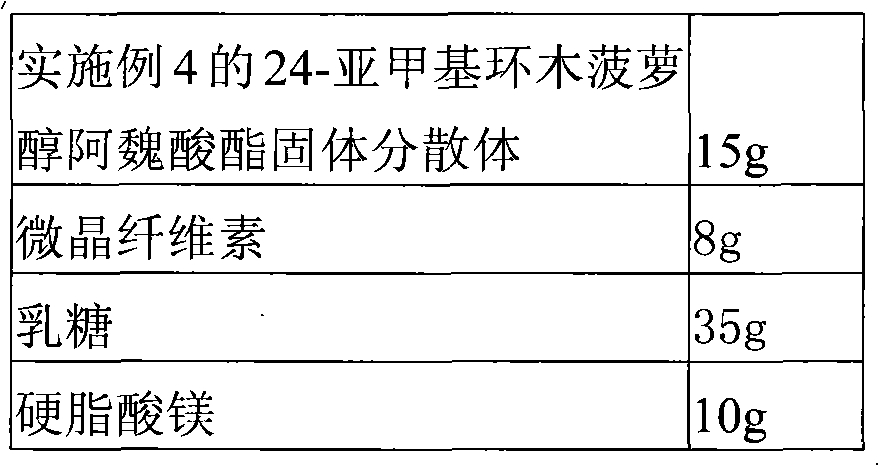
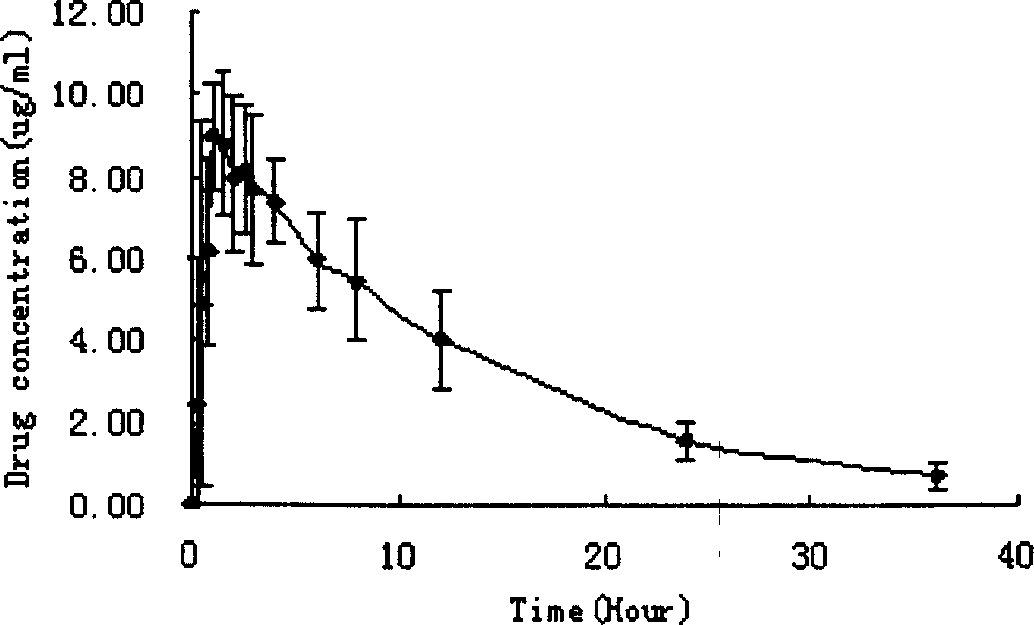
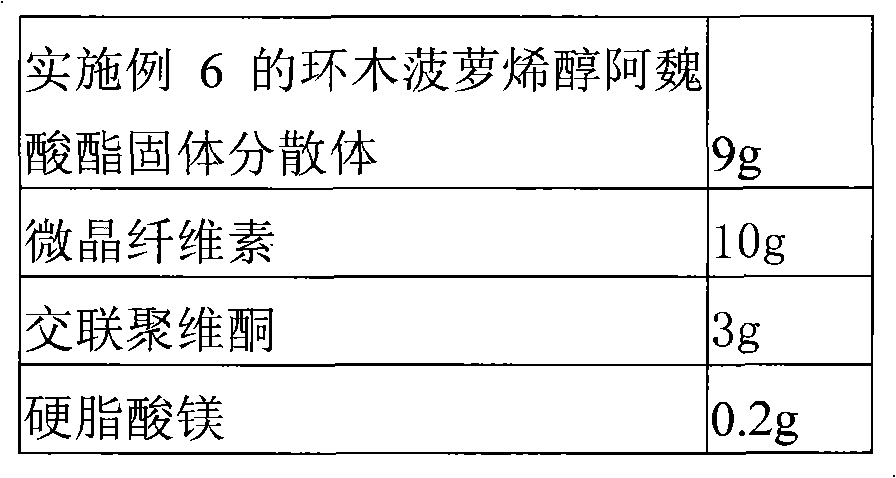
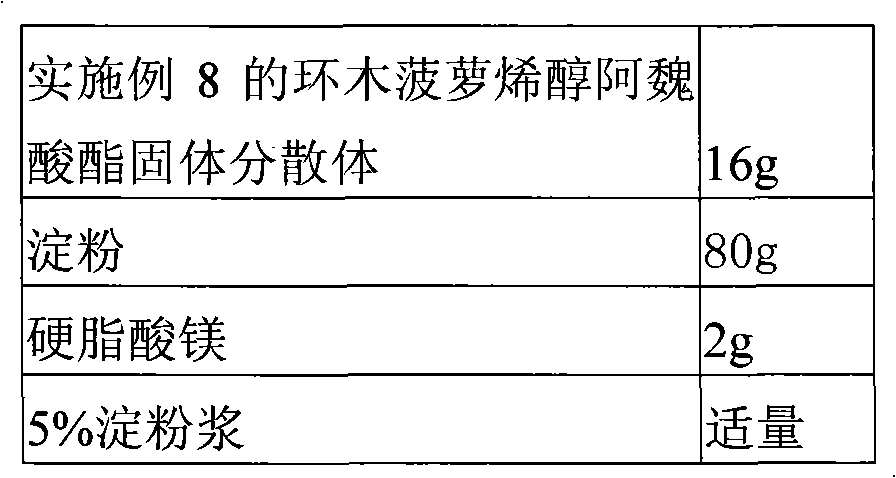
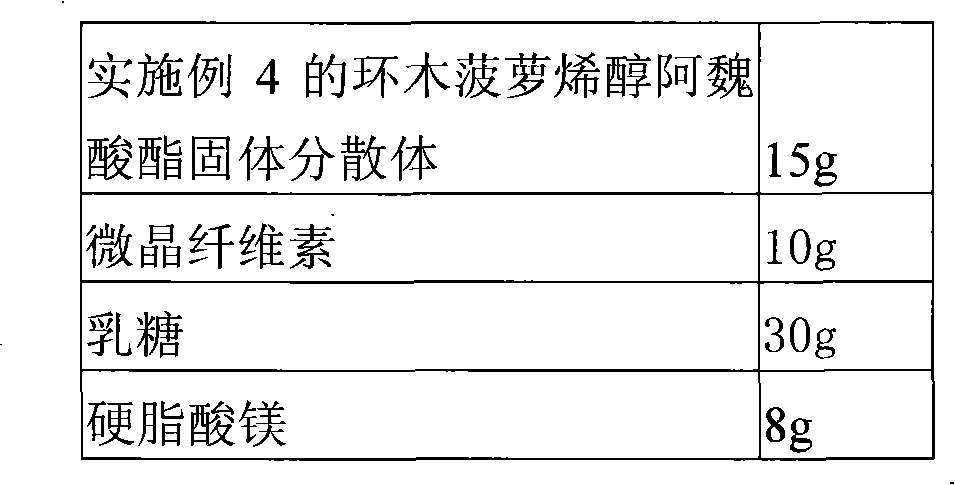
Solid dispersion of 24-methylene cycloartanol ferulic acid eater and preparation thereof
ActiveCN102058515AImprove in vitro dissolutionImprove dispersionPowder deliveryOrganic active ingredientsSolubilityDissolution
The invention discloses a solid dispersion of 24-methylene cycloartanol ferulic acid eater, comprising the 24-methylene cycloartanol ferulic acid eater and povidone as a carrier material. The invention further discloses a pharmaceutical preparation and a preparation method of the solid dispersion. Proved by experiments, the solid dispersion makes an obvious technical progress on technical indexes such as dissolution rate, stability, water-solubility, bioavailability and the like.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
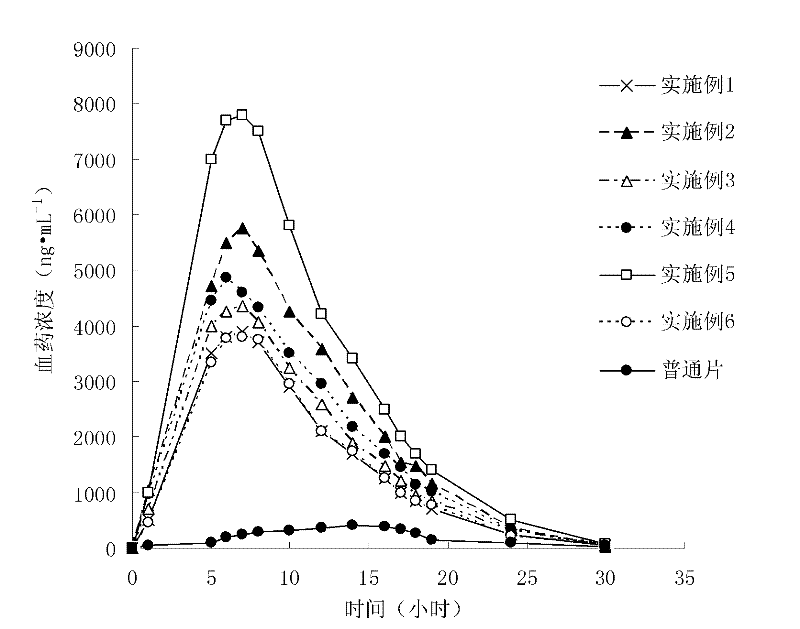
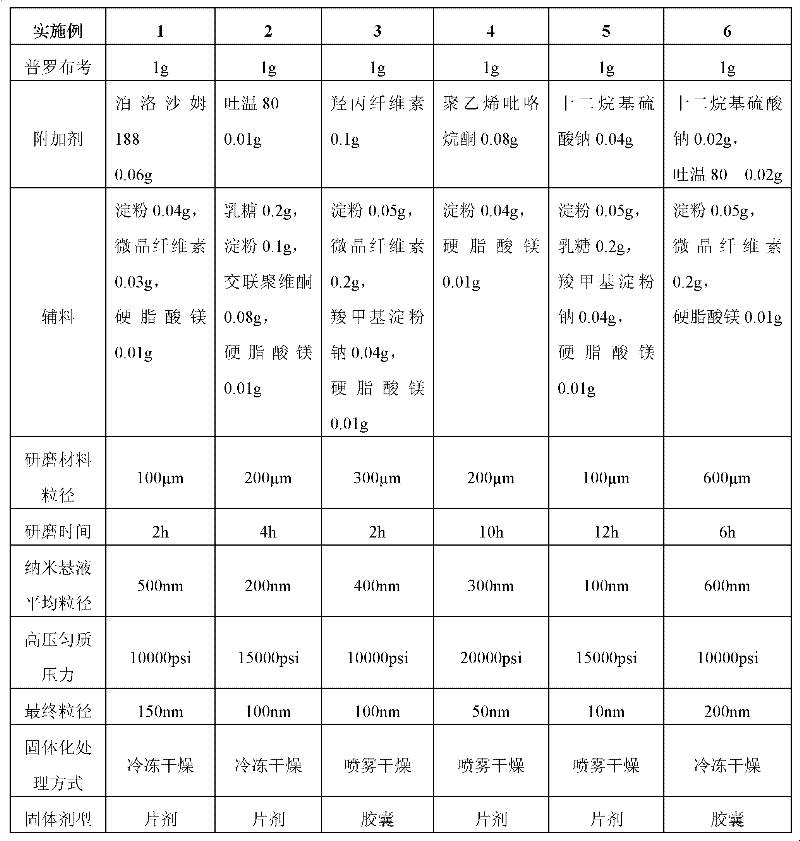
Probucol orally administered nanometer solid preparation and preparation method for same
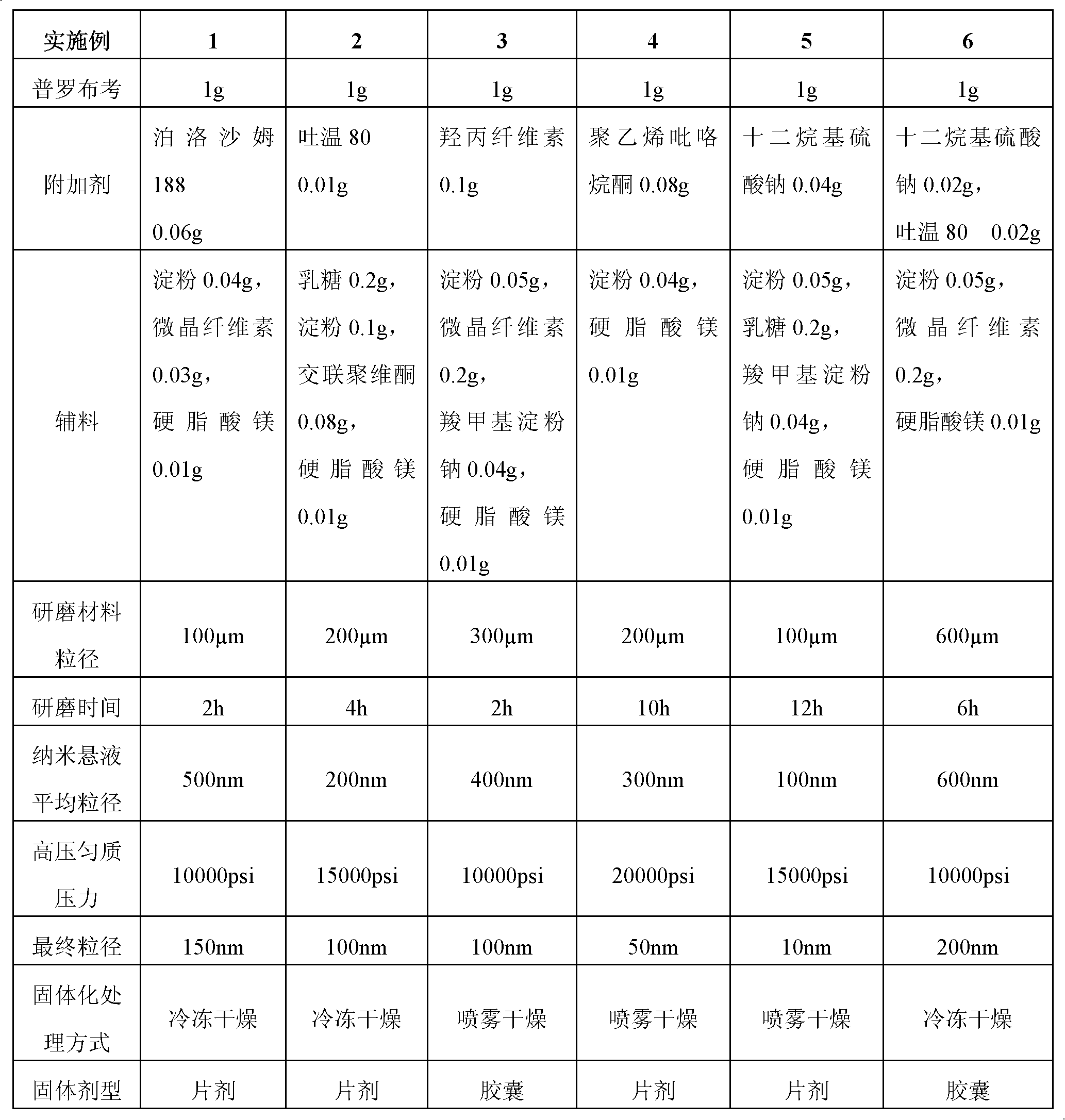
InactiveCN102475688AImprove in vitro dissolutionImprove bioavailabilityPowder deliveryMetabolism disorderDrugOral medication
The invention provides a probucol orally administered nanometer solid preparation, which comprises, by weight, 1 part of probucol, 0.01-0.1 part of additive and 0.05-0.4 part of auxiliary materials. The grain size of the probucol ranges from 10nm to 200nm. The invention further provides a preparation method for the probucol orally administered nanometer solid preparation. The preparation method includes that the probucol and water liquor with 5% of additive are prepared into nanometer suspension with the grain size ranging from 100nm to 600nm by a wet grinding method; the nanometer suspension is homogenized into nanometer grains with the grain sizes ranging from 10nm to 200nm by a high-pressure homogenizing machine; and the nanometer suspension which is homogenized is solidified and is added with the auxiliary materials to be prepared into the orally administered nanometer solid preparation. Compared with a common probucol tablet, the preparation has the advantages that dissolution in vitro of the preparation is increased by 20-30 times, and bioavailability after oral administration is improved by 10-20 times. In addition, the drug loading rate of the preparation is high, and the preparation can meet the requirements of tablet weight clinically needed by high-dose probucol administration. Besides, the preparation is stable, and the dissolution of the preparation can be kept unchanged within two years.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
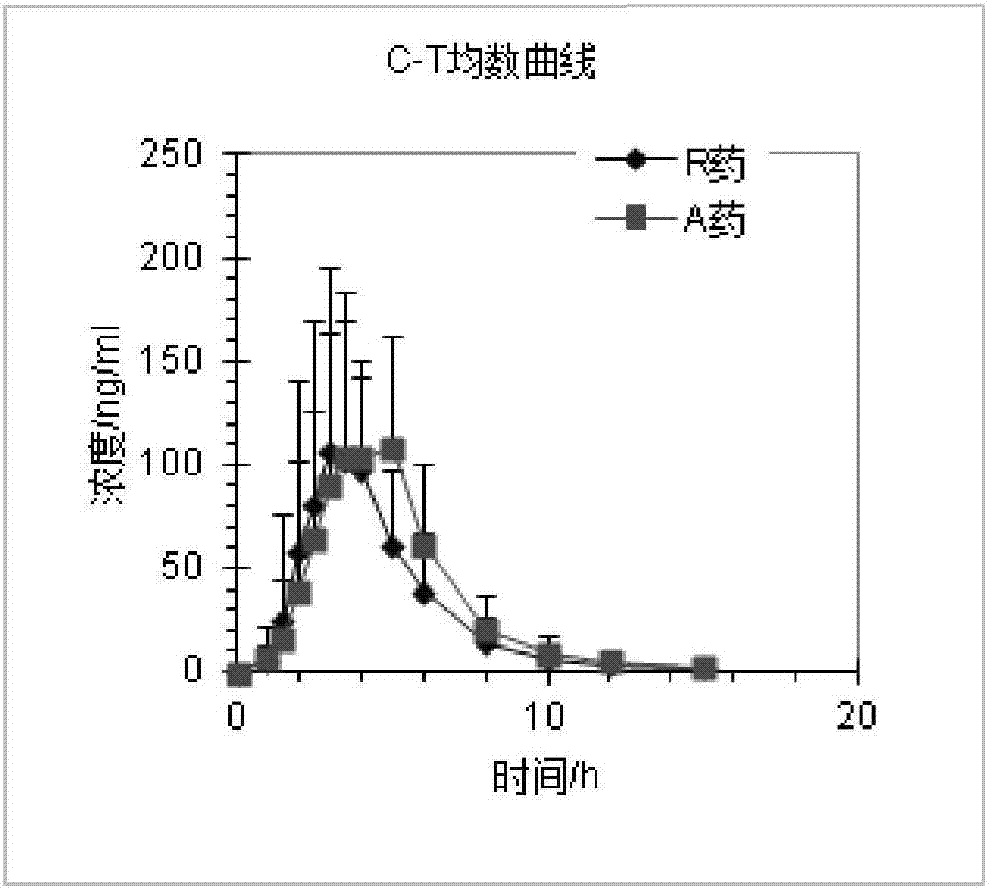
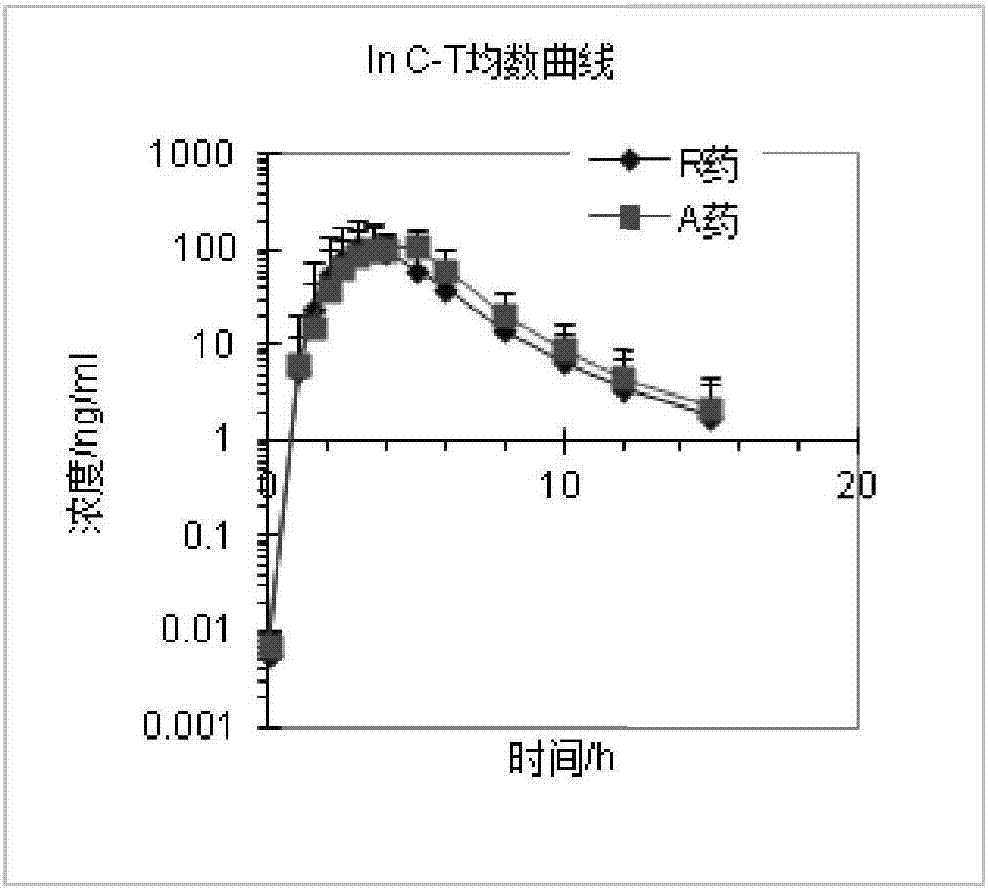
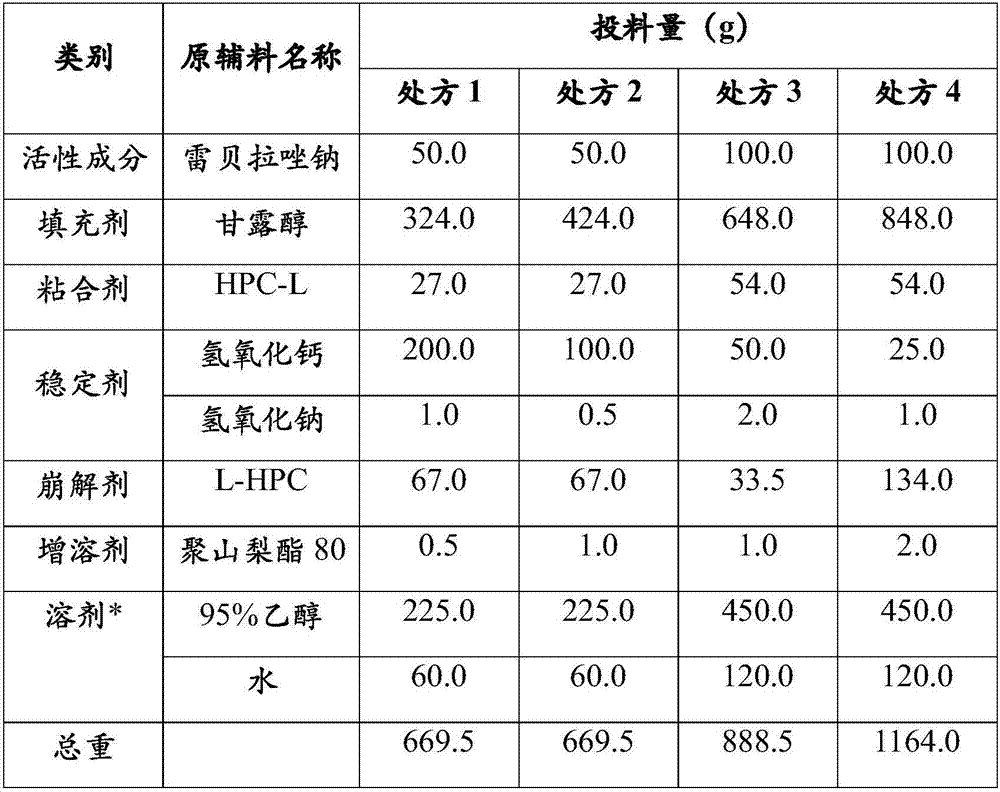
Preparation process of rabeprazole sodium enteric capsules
ActiveCN107019680AIncrease productivityImprove bioavailabilityOrganic active ingredientsDigestive systemCelluloseHard Capsule
The invention discloses a preparation process capable of improving bioavailability of rabeprazole sodium enteric capsules. The rabeprazole sodium enteric capsules prepared by the invention are prepared from rabeprazole sodium enteric mini-pills and hard capsule shells, wherein each rabeprazole sodium enteric mini-pill comprises a drug-loaded pill core, an isolation layer and an enteric layer; each drug-loaded pill is prepared from rabeprazole sodium, mannitol, low substituted hydroxypropy cellulose, high substituted hydroxypropyl cellulose L, calcium hydroxide, sodium hydroxide, and tween 80. Each isolation layer is prepared from ethyl cellulose, high substituted hydroxypropyl cellulose L and magnesium stearate; the enteric layer is prepared from acrylic resin, triethyl citrate and talcum powder. According to the preparation process, the production efficiency of the rabeprazole sodium enteric capsules can be improved, the produced rabeprazole sodium enteric capsules are uniform in quality, good in stability and high in dissolution in vitro; moreover, the bioavailability of rabeprazole sodium enteric capsules can be obviously improved, and the rabeprazole sodium enteric capsules have good market prospect.
Owner:珠海润都制药股份有限公司
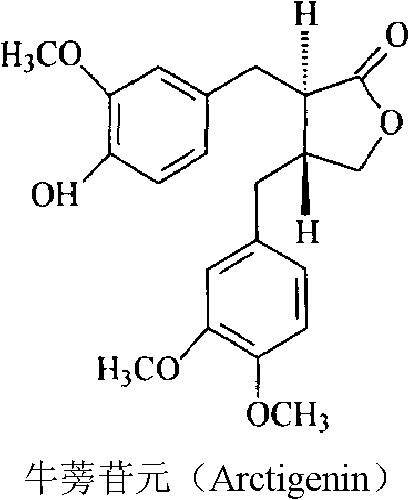
Solid dispersion of arctigenin and oral solid preparation
ActiveCN102885781APromote absorptionImprove the deficiency of low bioavailabilityAntibacterial agentsOrganic active ingredientsOral medicationIrritation
The invention relates to a solid dispersion of arctigenin and an oral solid preparation. The solid dispersion consists of the arctigenin and a carrier material, wherein the weight ratio of the arctigenin to the carrier material is 1:1-1:50. The defects of poor oral administration absorption and low bioavailability of the arctigenin are overcome, stability of the arctigenin is improved, objectionable odor and irritation of medicaments are concealed, and a preparation technology is simple and suitable for industrialized production.
Owner:LUNAN BETTER PHARMA
Breviscapine composition nano-particle and preparation method thereof
InactiveCN103690489AImprove solubilityImprove in vitro dissolutionOrganic active ingredientsPowder deliveryPrillNanoparticle
The invention belongs to the field of pharmacy, and relates to a breviscapine composition nano-particle and a preparation method thereof. The provided novel breviscapine composition nano-particle is prepared by a solution containing the breviscapine, a polymer, a surfactant and a solvent with the adoption of an electrostatic spraying technology. The composition preparation device is simple, the parameters are controllable, the drug loading capacity is high, , the direct and continuous nano-particles with uniform particle size distribution can be obtained, the specific surface area is increased, and the dissolution in vitro and bioavailability of the breviscapine are improved remarkably.
Owner:NANJING DACHUANG BIOLOGICAL SCI & TECH CO LTD
Oral gatifloxacin disintegrant and its preparing process
InactiveCN1857228APromote dissolutionQuickly exert the therapeutic effect of the whole bodyAntibacterial agentsOrganic active ingredientsAdhesiveDentistry
The present invention provides an oral gatifloxacin disintegrant and its preparation process. The oral gatifloxacin disintegrant contains gatifloxacin in 20-75 wt% and medicinal supplementary material in 25-80 wt%, and the medicinal supplementary material includes one or several of disintegrating agent, stuffing, wetting adhesive, lubricant and coating material. The preparation process of the oral gatifloxacin disintegrant includes coating medicine powder or medicine carrying micro pill and tabletting. The oral gatifloxacin disintegrant of the present invention has the advantages of fast medicine release, increased absorbing points and less local excitation on gastrointestinal tract and is suitable for taking without using water and by patient with dysphagia.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Cycloartenyl ferulate solid dispersion and preparation thereof
ActiveCN102058538AImprove in vitro dissolutionImprove dispersionOrganic active ingredientsPowder deliveryCycloartenyl ferulateSolubility
The invention discloses cycloartenyl ferulate solid dispersion, which comprises cycloartenyl ferulate and povidone serving as a carrier material. The invention further discloses a medicinal preparation and a preparation method of the solid dispersion. Experiments prove that the solid dispersion makes obvious technical progress in technical indexes such as dissolution rate, stability, water solubility, bioavailability and the like.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
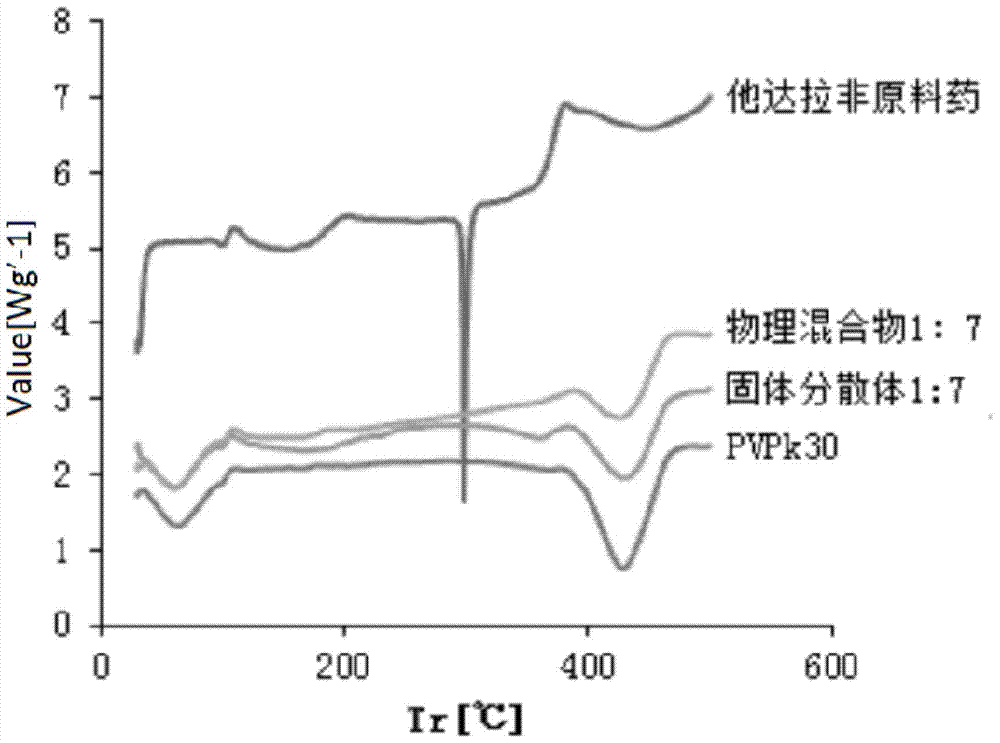
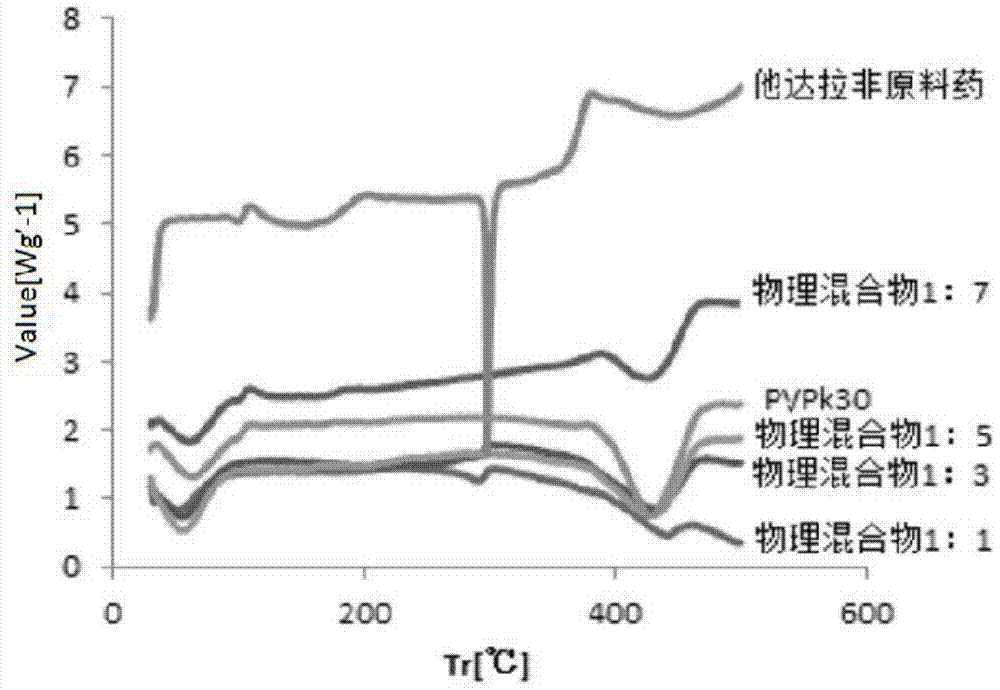
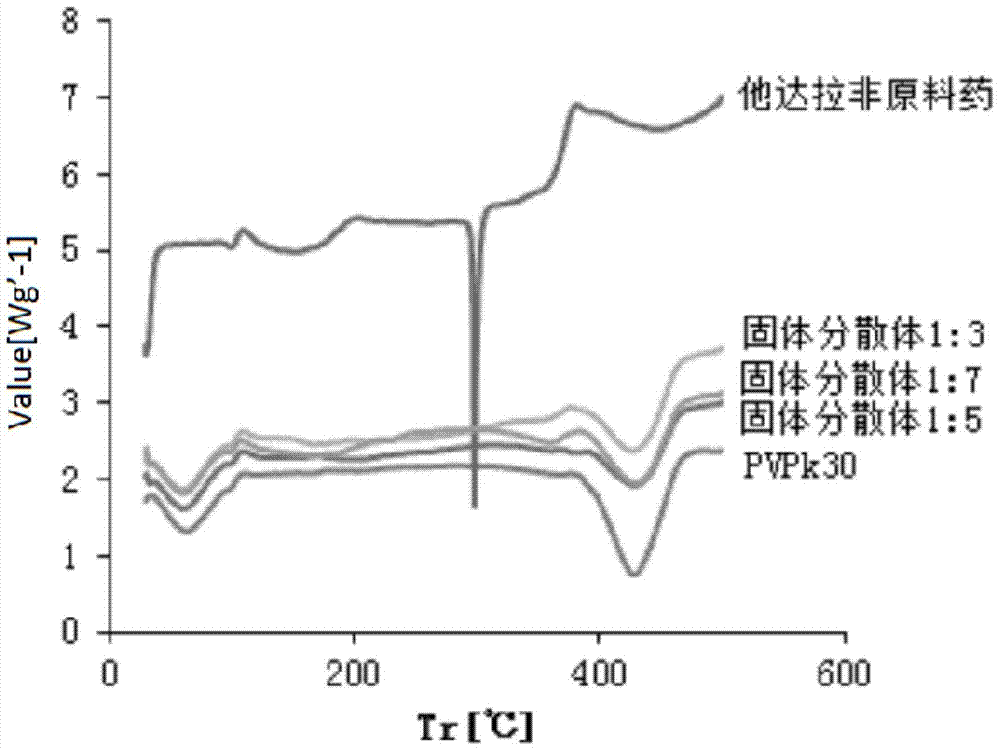
Tadalafil solid dispersion and its tablet
InactiveCN104188912BImprove solubilityImprove in vitro dissolutionOrganic active ingredientsPowder deliveryTadalafilEvaporation
The invention discloses a preparation method of tadalafil solid dispersion: dissolving tadalafil and a carrier material in absolute ethanol, and evaporating under reduced pressure at 50-90°C to obtain it; the tadalafil and The mass ratio of the carrier material is 1:0.5-1:13; the carrier material is selected from one or both of polyvinylpyrrolidone K30 and poloxamer. The present invention also provides a tadalafil solid dispersion tablet: made of tadalafil solid dispersion, filler, disintegrant, lubricant, wherein, the amount of filler accounts for 40% of the total weight of the tablet % to 70%; the amount of disintegrant accounts for 0.5% to 10% of the total weight of the tablet; the amount of lubricant accounts for 0.1% to 5% of the total weight of the tablet. The tadalafil solid dispersion and the tablet of the invention improve the in vitro dissolution rate of the tadalafil, and improve the disadvantages of poor oral absorption and low bioavailability.
Owner:SHANDONG UNIV
Solid sirolimus self-microemulsion preparation and preparation method thereof
InactiveCN106727317AImprove solubilityHigh dissolution rateOrganic active ingredientsAntipyreticPolyethylene glycolMesoporous silica
The invention discloses a solid sirolimus self-microemulsion preparation and a preparation method thereof. The solid sirolimus self-microemulsion preparation is prepared by adding a liquid self-microemulsion preparation prepared from sirolimus, polyethylene glycol oleate glyceride, polyoxyethylene-35-castor oil and diethylene glycol monoethyl ether to mesoporous silica nanoparticles, wherein the weight ratio of the liquid self-microemulsion preparation to the mesoporous silica nanoparticles is (1:1) to (10:1). The liquid self-microemulsion preparation is solidified by adopting the mesoporous silica nanoparticles, so that the disadvantages that the liquid self-microemulsion preparation is not easy to transport, store and take are reduced; compared with a commercially available tablet, the prepared solid sirolimus self-microemulsion preparation is capable of significantly improving the in-vitro dissolution rate and the in vivo bioavailability of the sirolimus. The preparation technology is simple, feasible and low in cost and has certain economic benefits and wide application prospects.
Owner:FUZHOU GENERAL HOSPITAL OF NANJING MILITARY COMMAND P L A
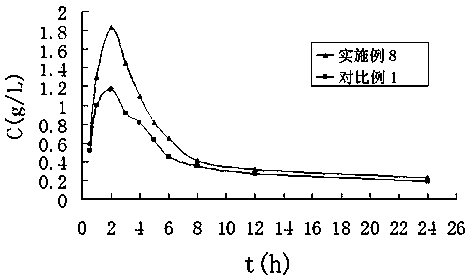
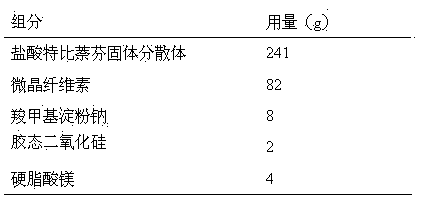
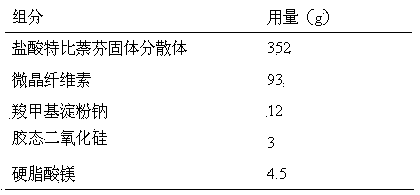
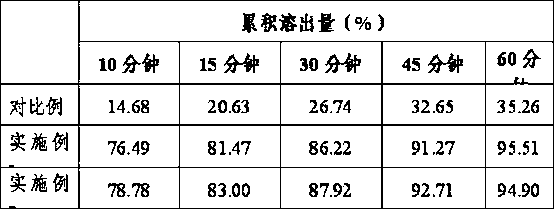
Terbinafine hydrochloride solid dispersoid and tablet thereof
ActiveCN102641252BImprove in vitro dissolutionReduce dosageOrganic active ingredientsAntimycoticsBULK ACTIVE INGREDIENTPharmaceutical Substances
The invention relates to a terbinafine hydrochloride solid dispersoid, which is composed of terbinafine hydrochloride and carrier materials, wherein the proportion by weight between the terbinafine hydrochloride and the carrier materials is 1 / 2-1 / 15. The invention further relates to a tablet prepared by the terbinafine hydrochloride solid dispersoid. Compared with the prior art, the terbinafine hydrochloride tablet has the advantages of (1) improving dissolution in vitro of terbinafine hydrochloride preparation; (2) solving the problem that the terbinafine hydrochloride is poor in oral administration absorption and low in biological availability; (3) covering harmful odor and irritation of the terbinafine hydrochloride; (4) keeping pesticide effects of large dose while reducing using quantity of active ingredients in the preparation per unit, and reducing drug side-effects; and (5) being simple in preparation process and suitable for industrial production.
Owner:NANJING CHENGONG PHARM CO LTD
Preparation method of tolvaptan tablet
ActiveCN102366412BImprove bioavailabilityImprove in vitro dissolutionDigestive systemPill deliveryMagnesium stearateStearic acid
The invention discloses a preparation method of a tolvaptan tablet. The preparation method comprises the following steps: 1, mixing tolvaptan with high-substituted hydroxylpropyl cellulose according to a mass ratio of 1:0.2-0.6, crushing them, and sieving by a 60-100 mesh sieve; 2, dissolving throughs in a mixed solution of waterless ethanol and dichloromethane with a volume ratio of 1:1-4, carrying out spray drying to form powder, carrying out reduced pressure drying at 70-90DEG C until that the content of solvents residual in the sprayed powder is qualified; 3, crushing the sprayed powder, sieving by a 180-220 mesh sieve, accurately weighing the sprayed powder, lactose, microcrystalline cellulose and hydroxypropyl methylcellulose according to prescription amounts, uniformly mixing them, and adding a proper amount of water to prepare a soft material; and 4, sieving the soft material by a 15-25 mesh sieve, preparing a wet particle, drying the wet particle at 50-70DEG C until that the content of water in the wet particle is 2-4%, sieving the dried wet particle by a 15-25 mesh sieve, granulating, adding a prescription amount of magnesium stearate, uniformly mixing, and tabletting. The preparation method of the invention has the advantages of ingenious conception, low production cost, high in vitro dissolubility of the prepared tolvaptan tablet, and good biological availability and clinic curative effect of the tolvaptan tablet.
Owner:SICHUAN BAILI PHARM CO LTD
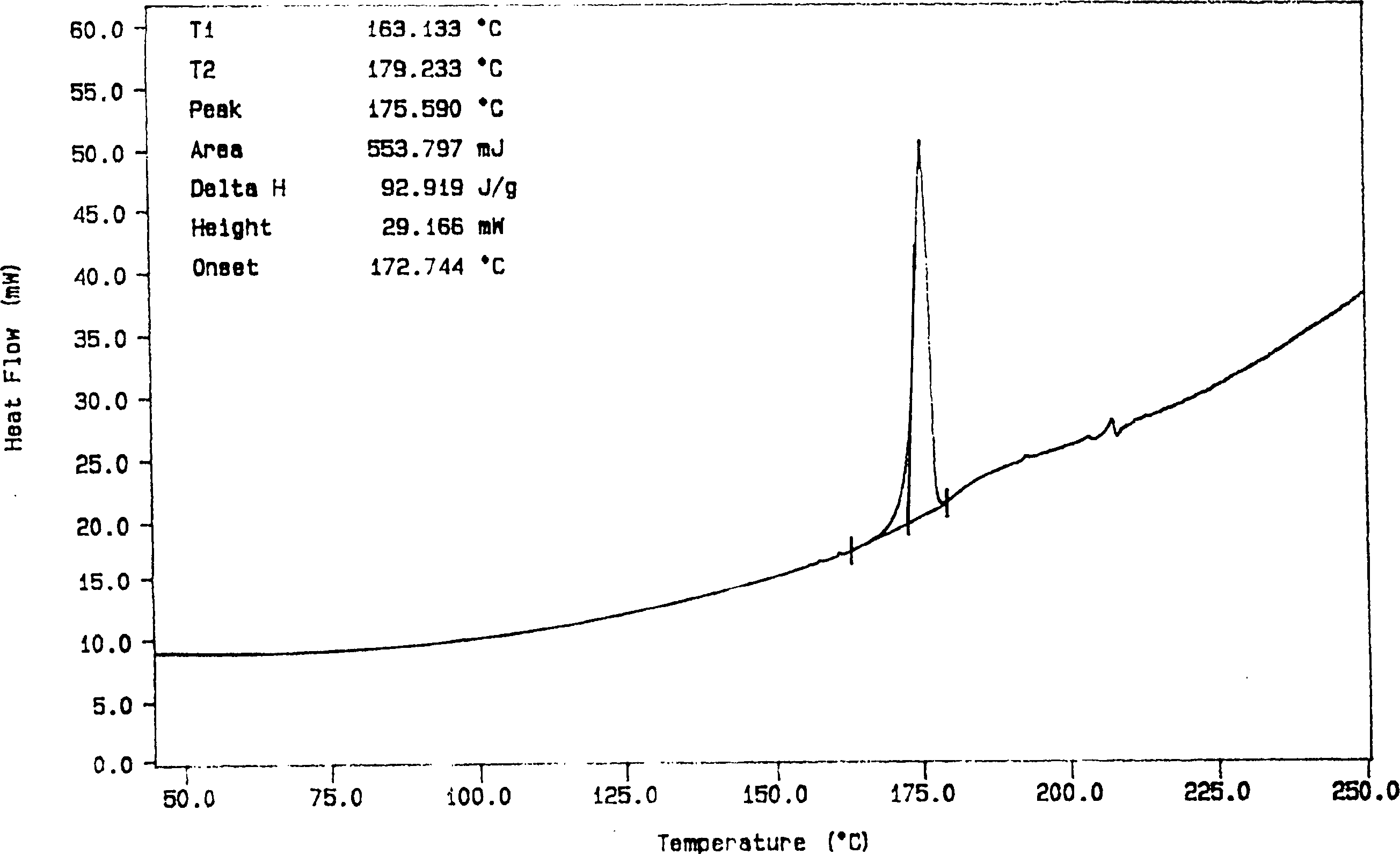
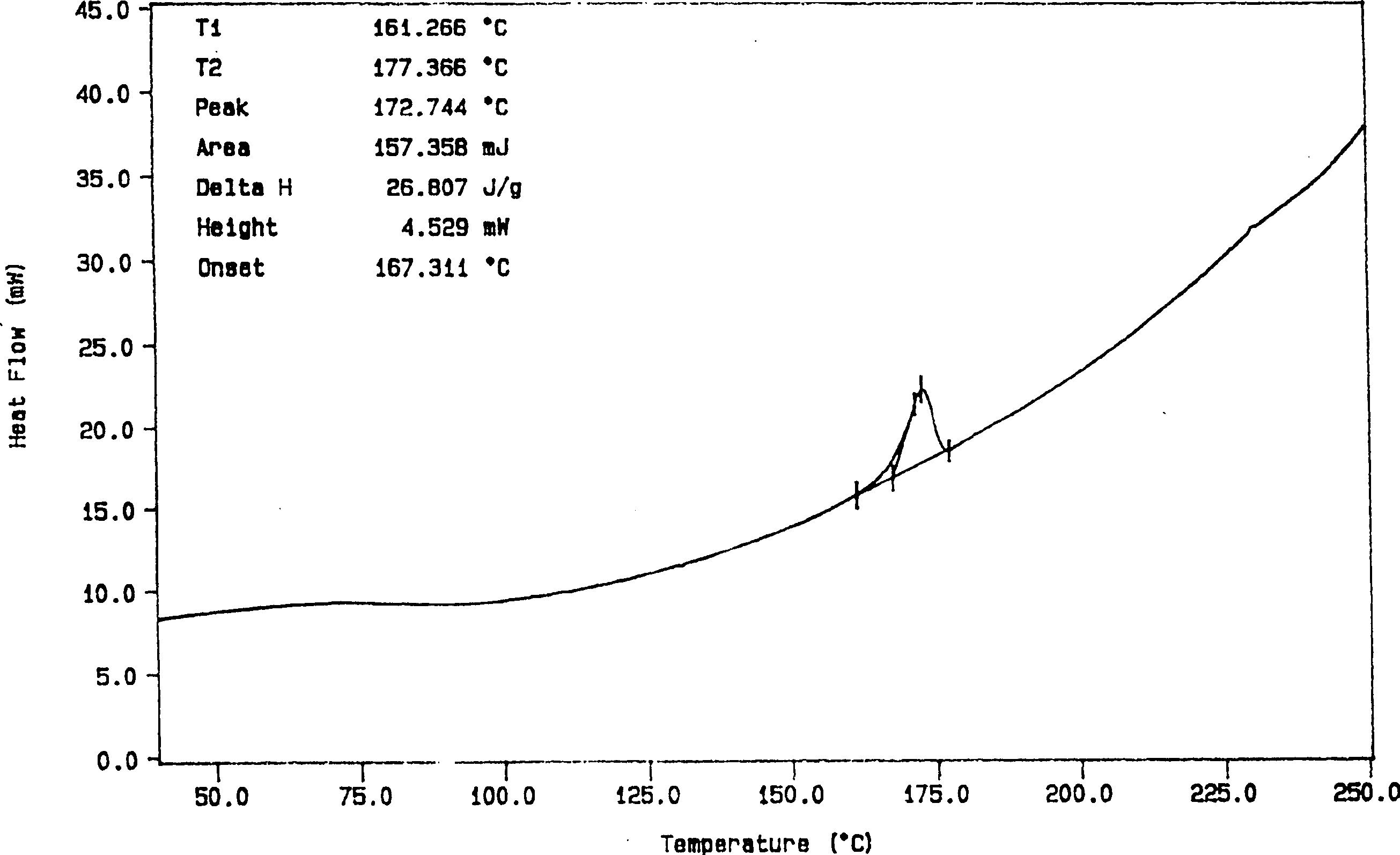
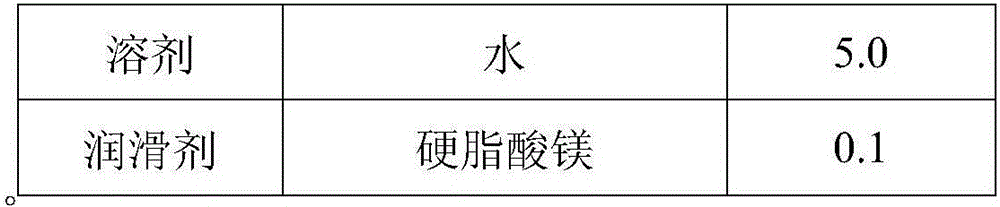
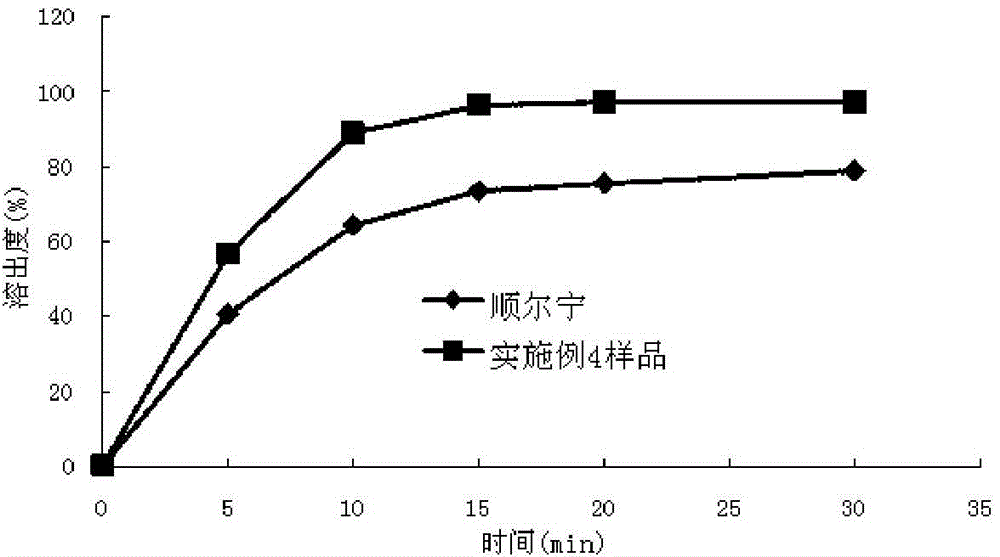
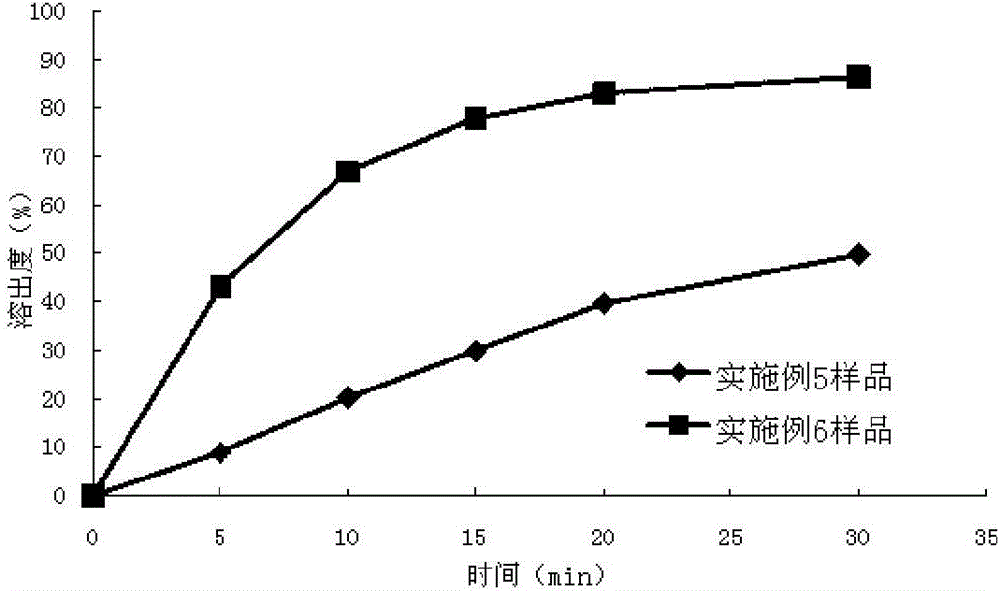
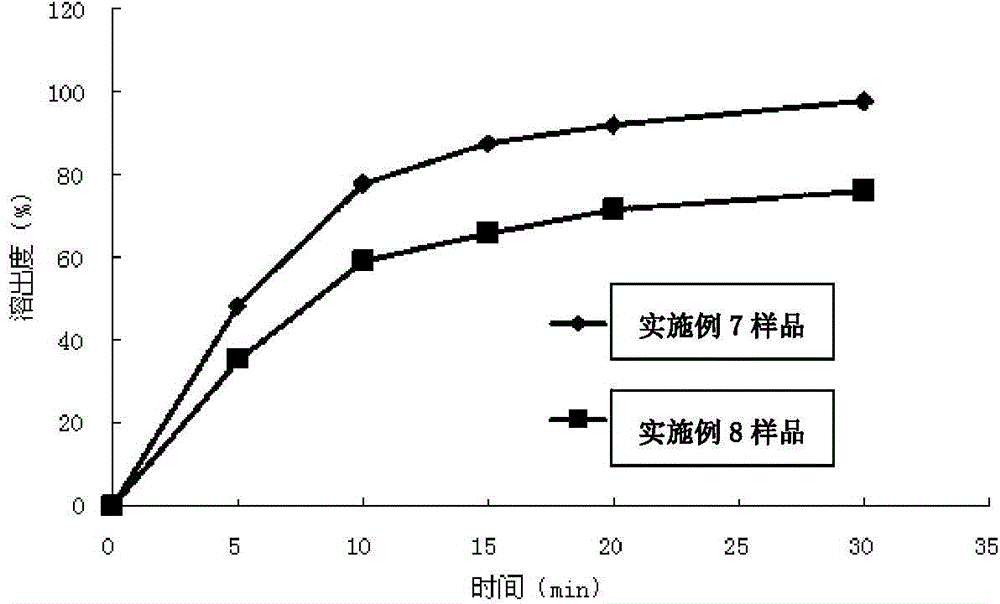
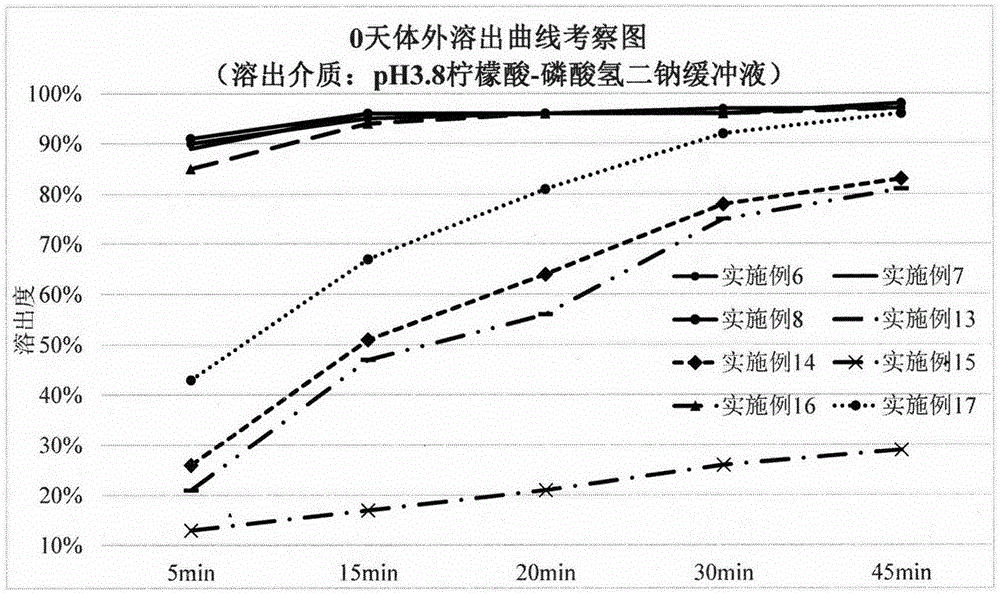
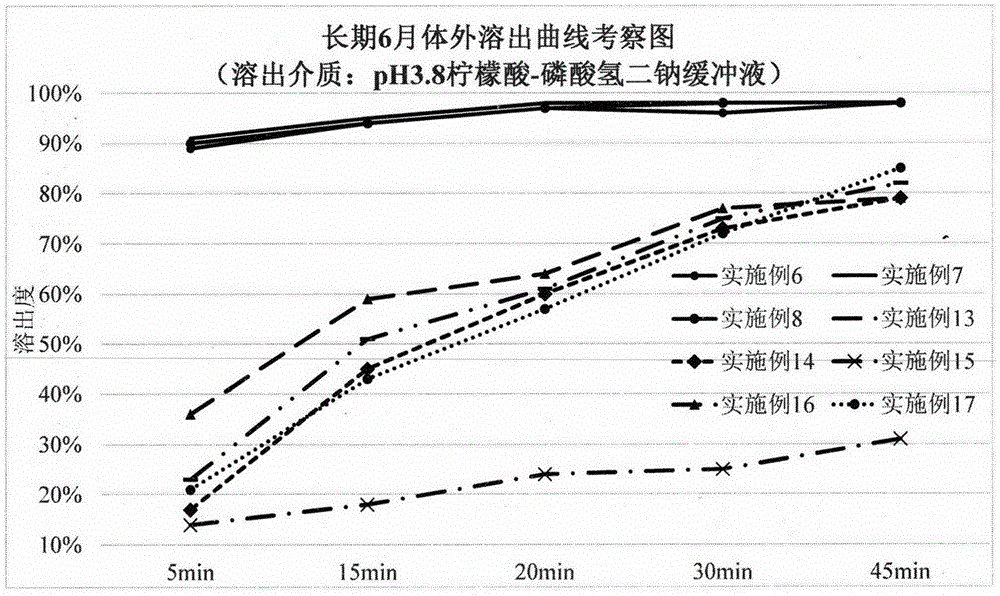
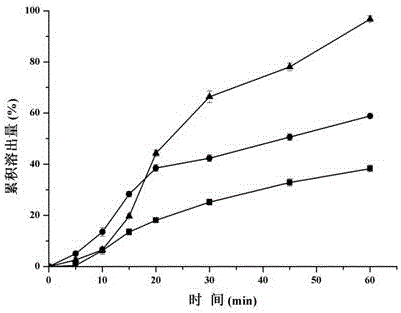
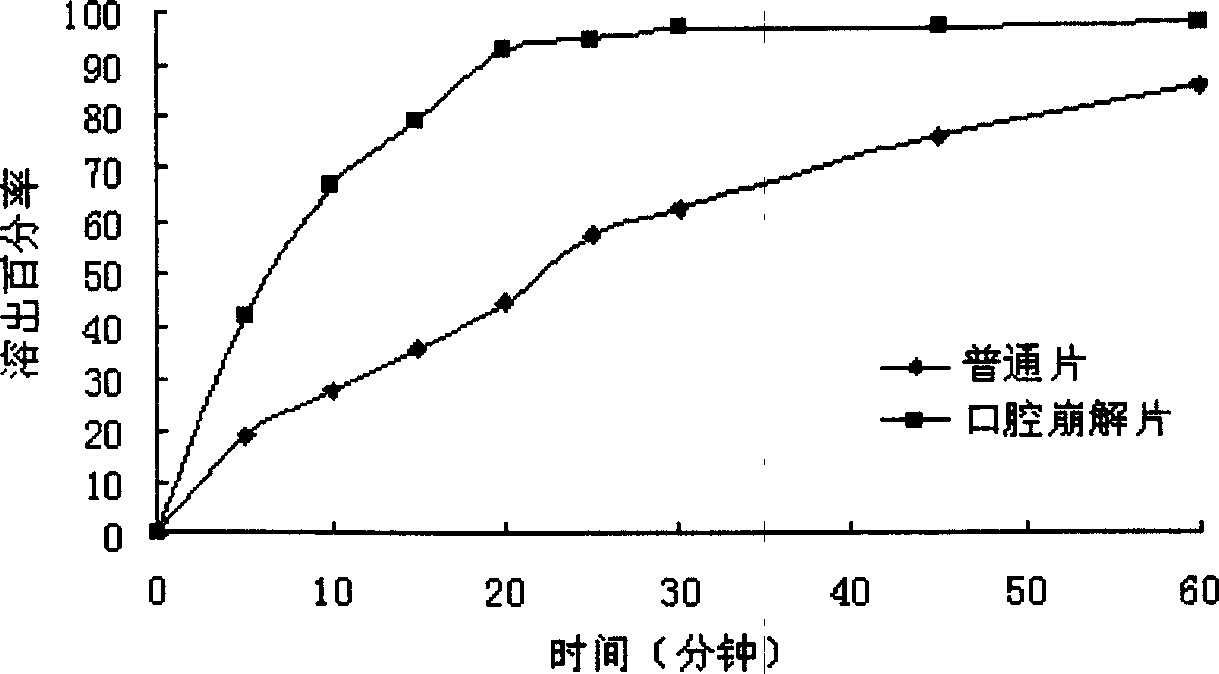
Solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid
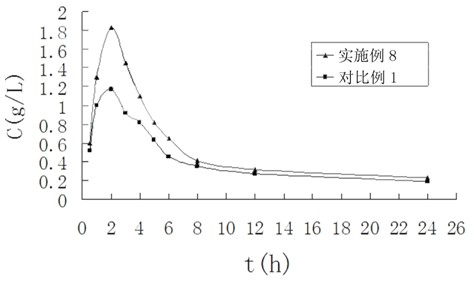
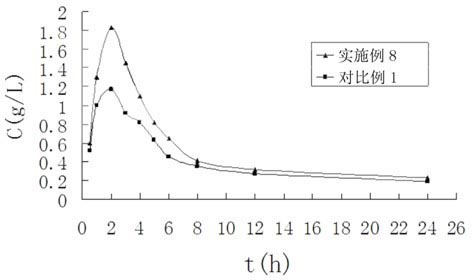
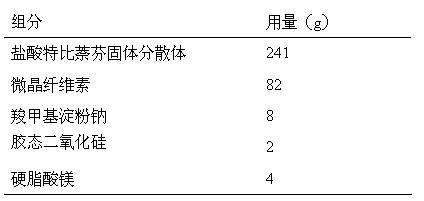
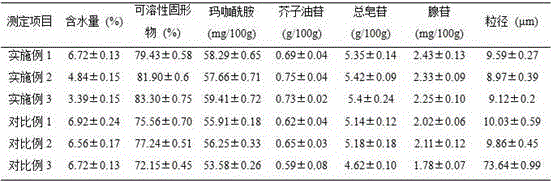
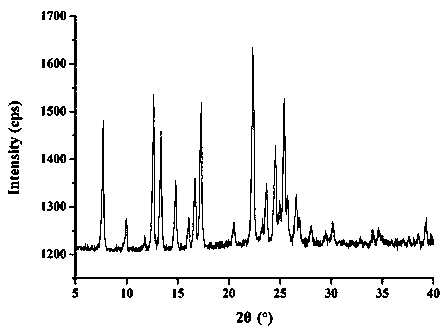
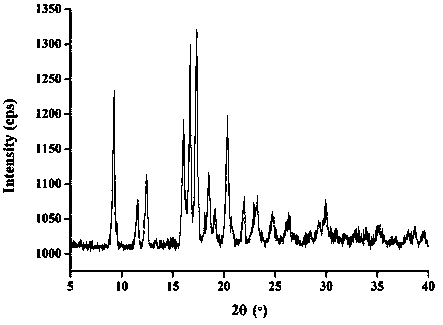
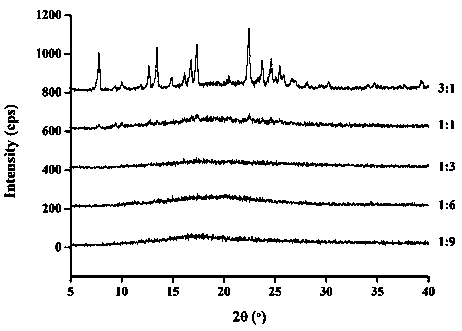
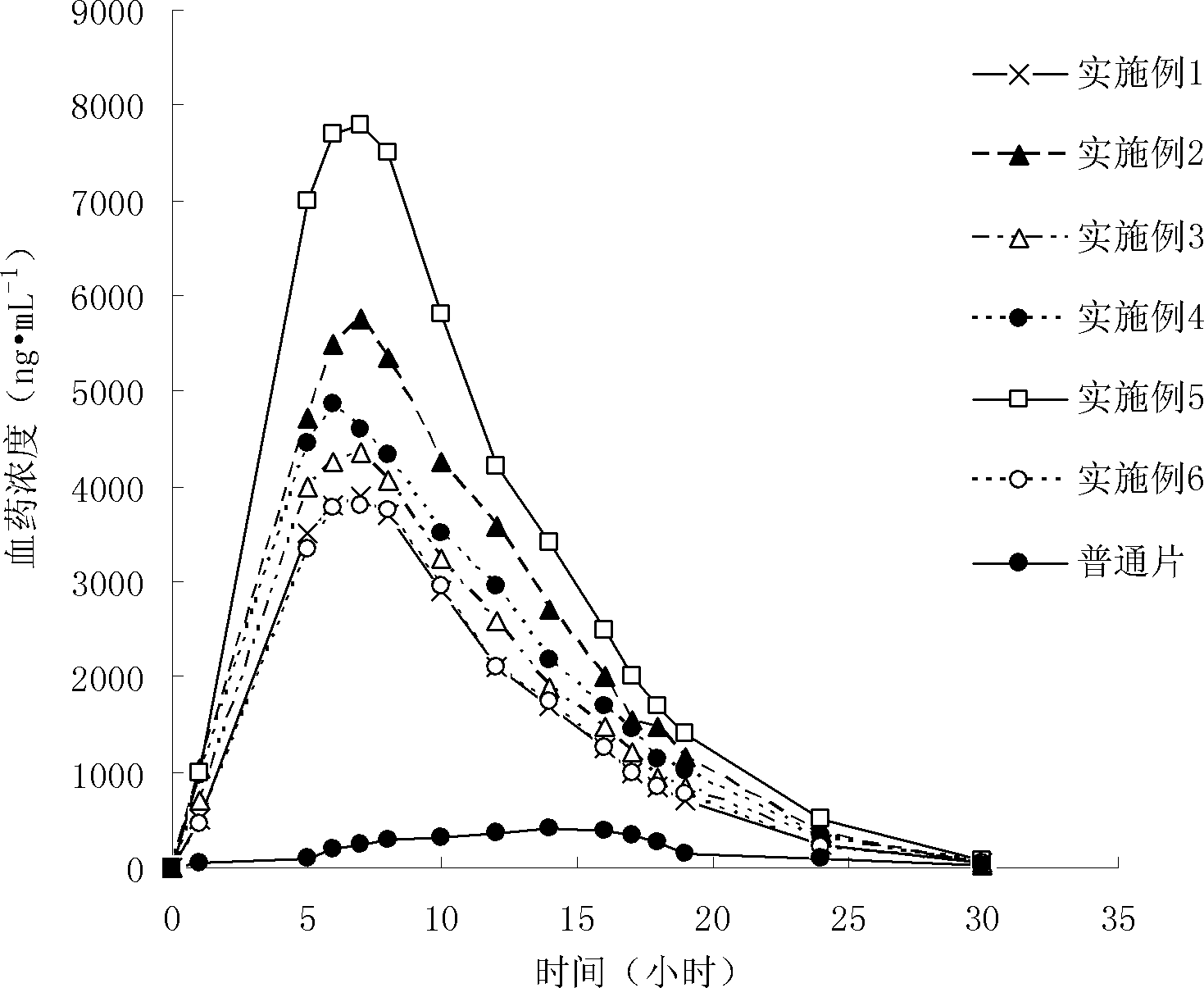
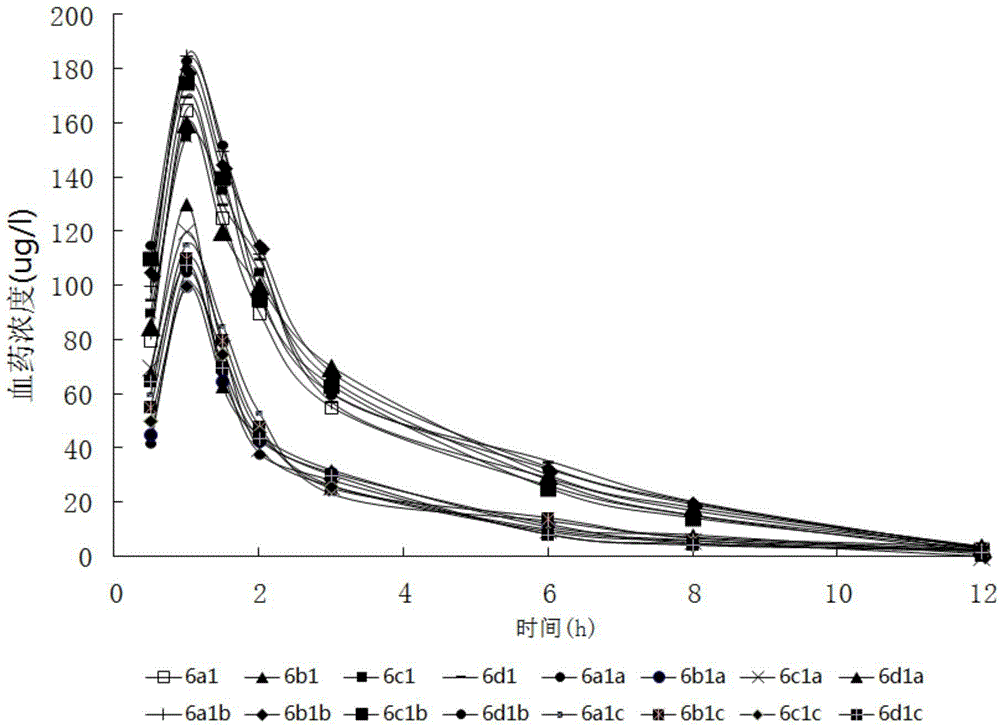
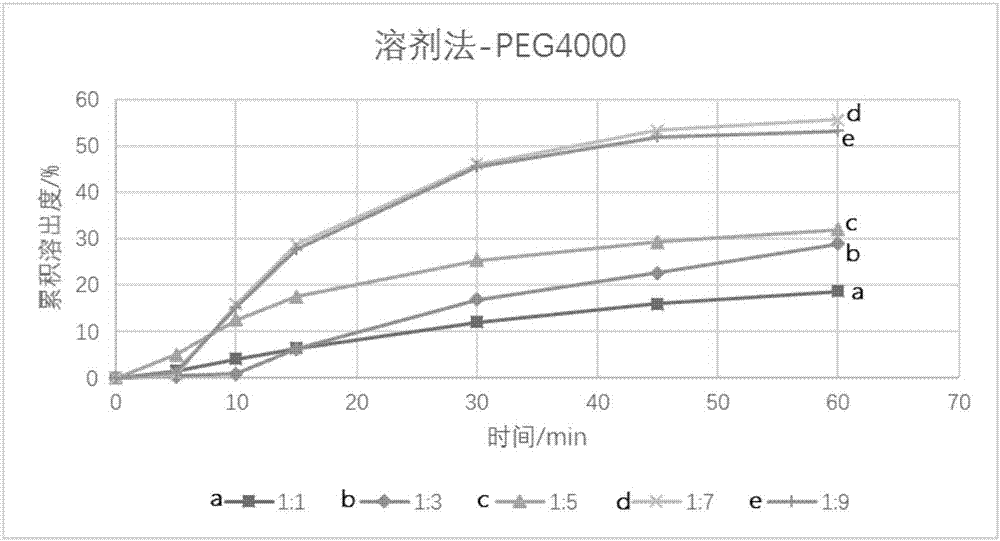
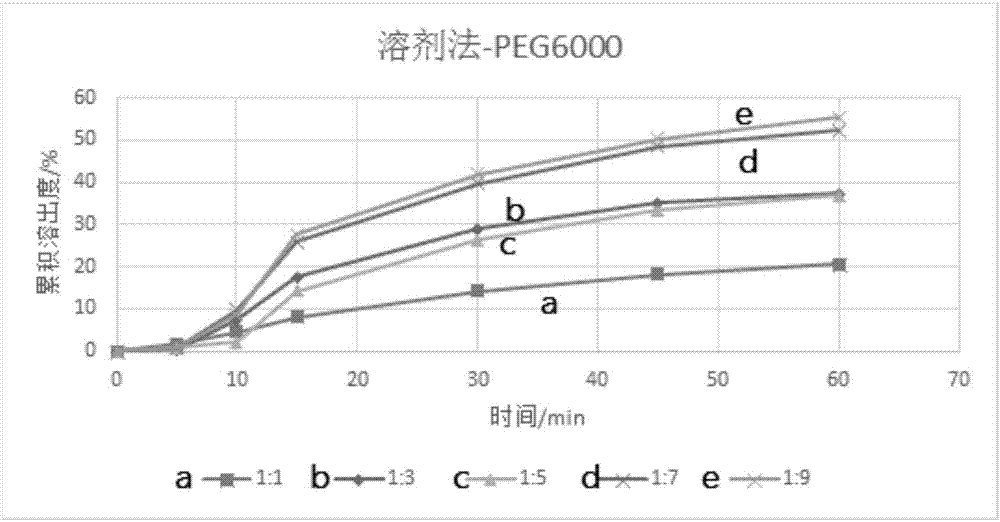
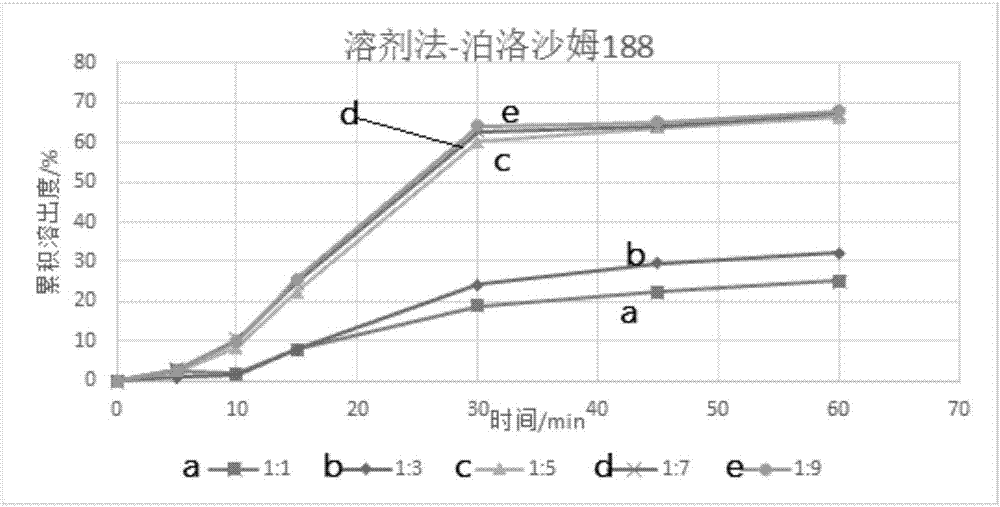
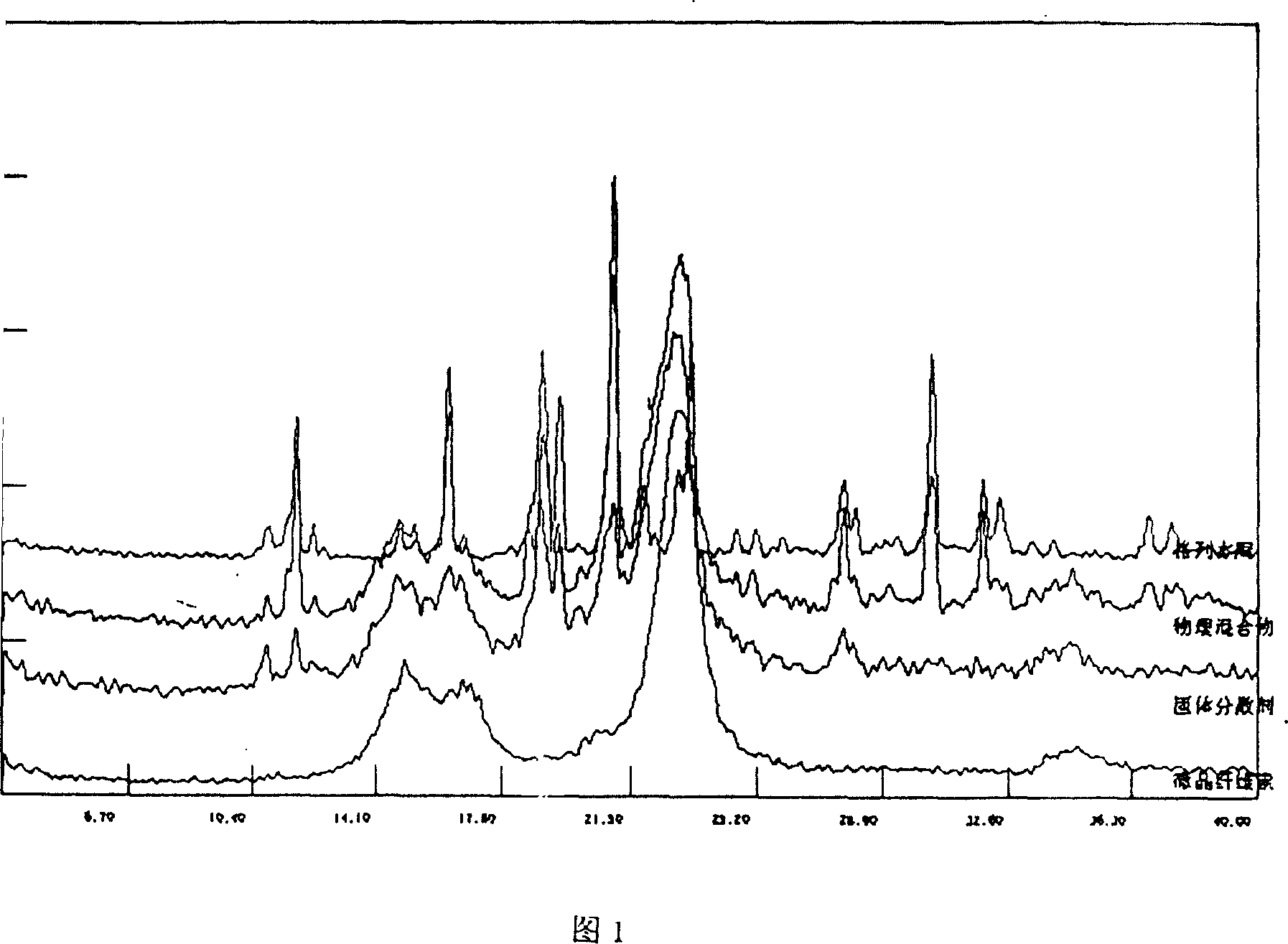
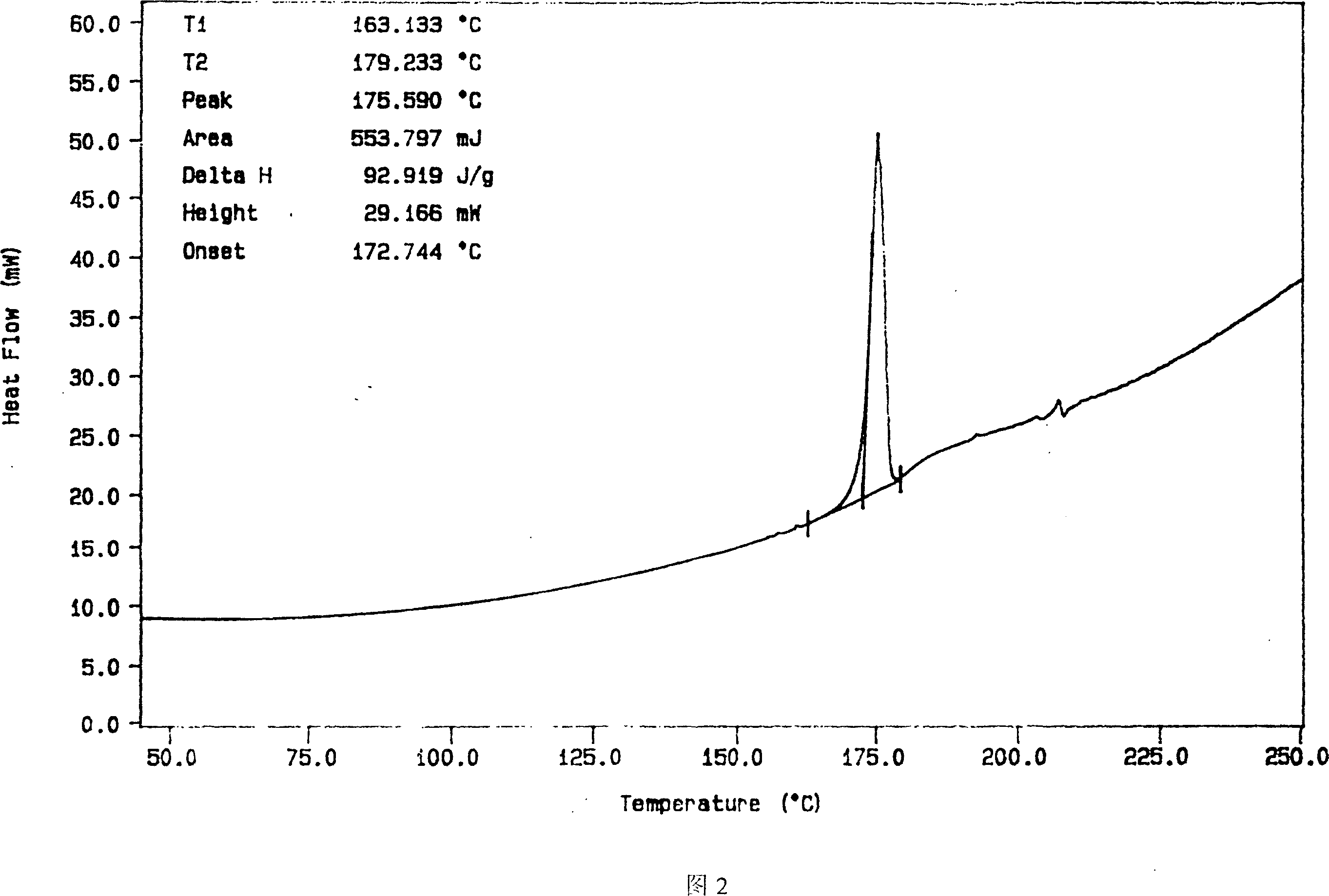
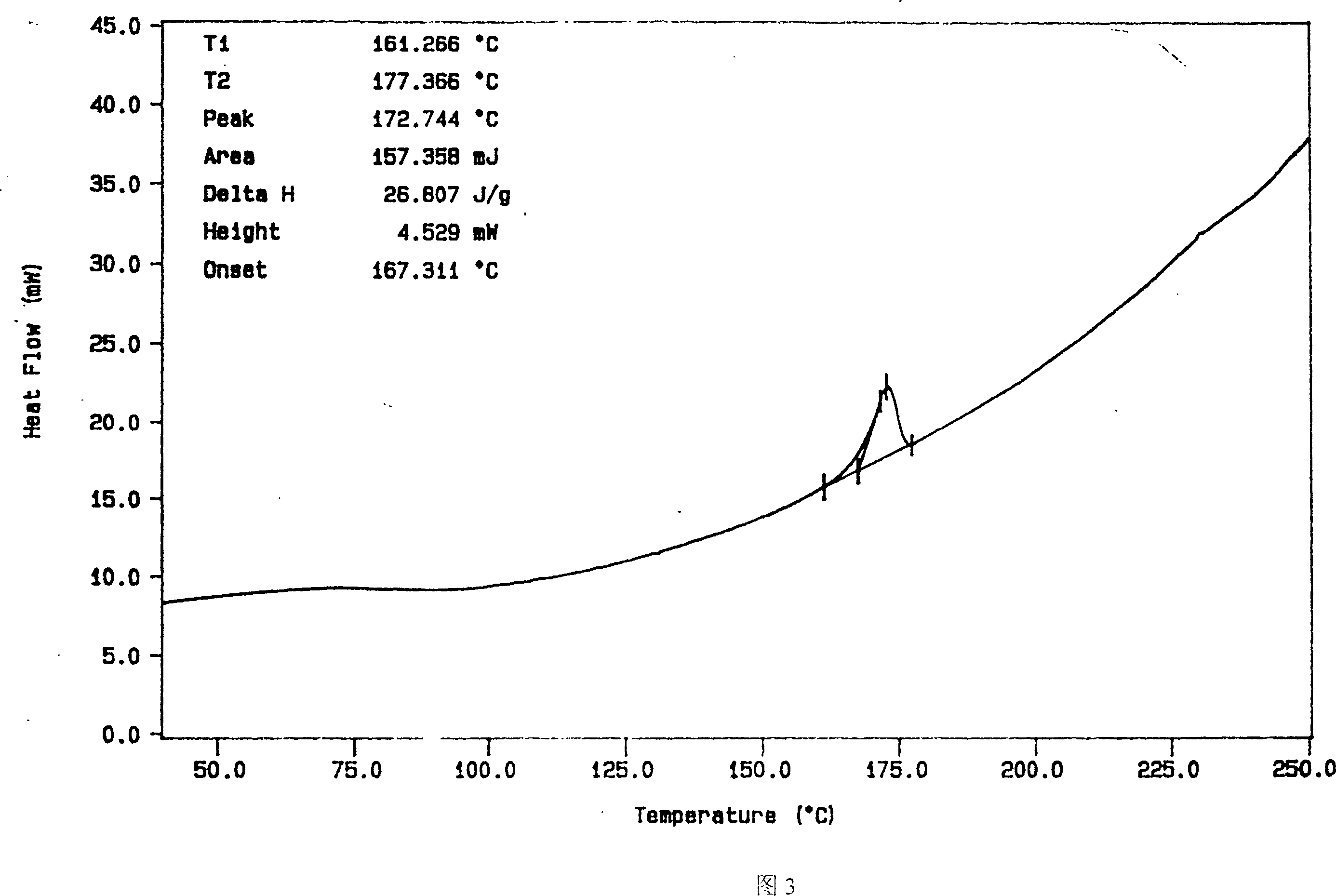
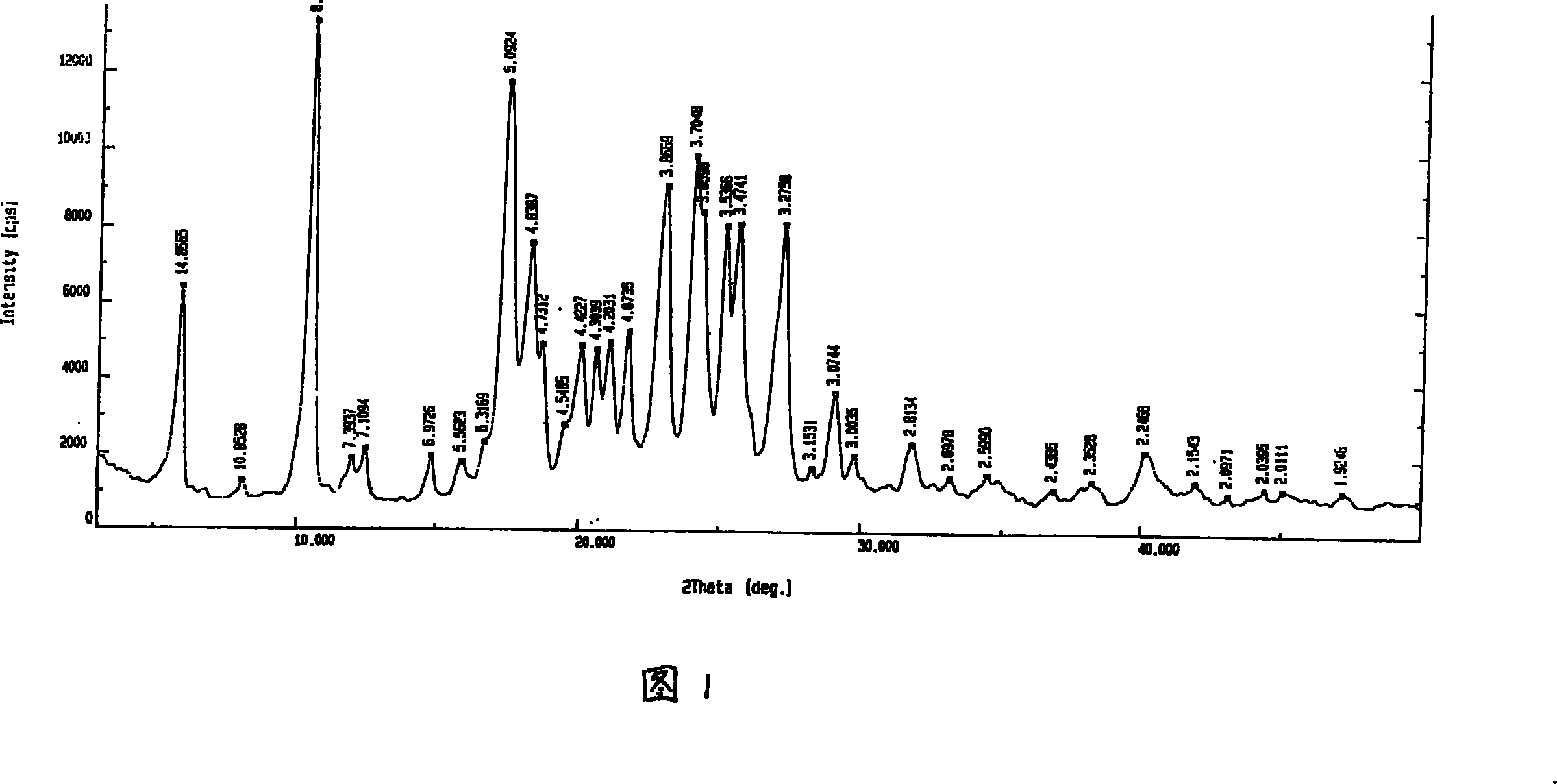
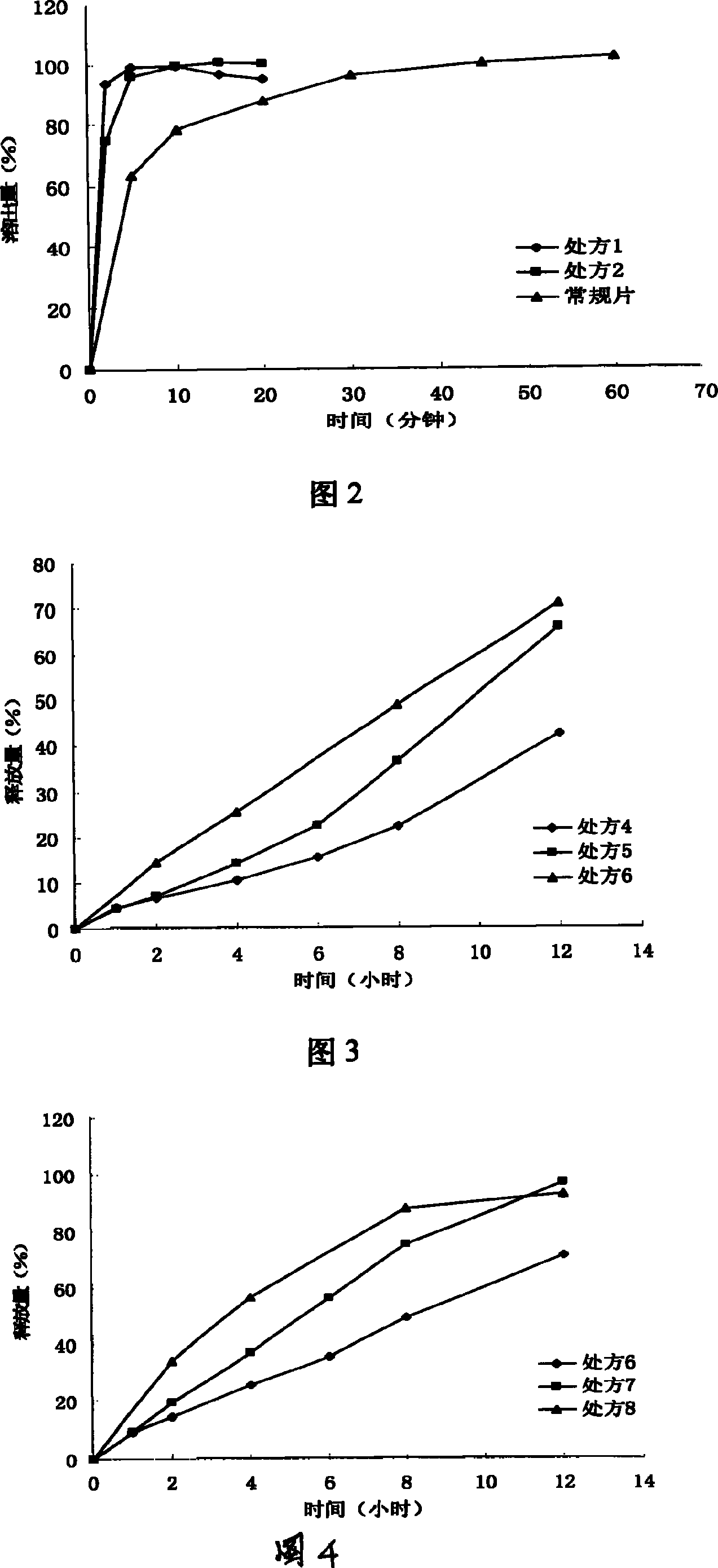
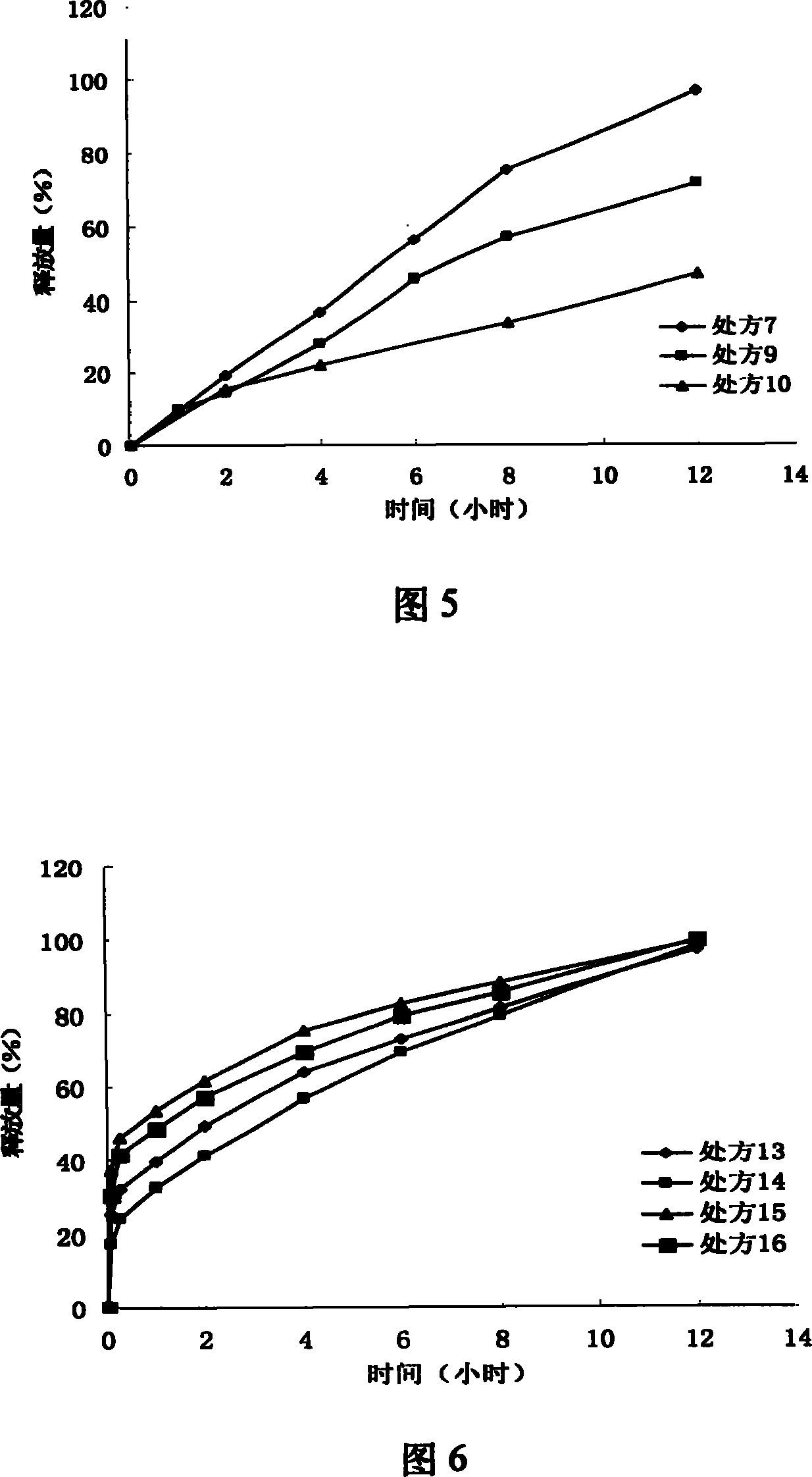
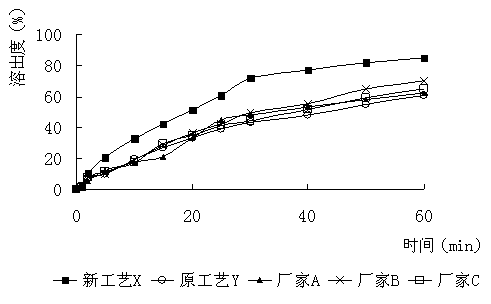
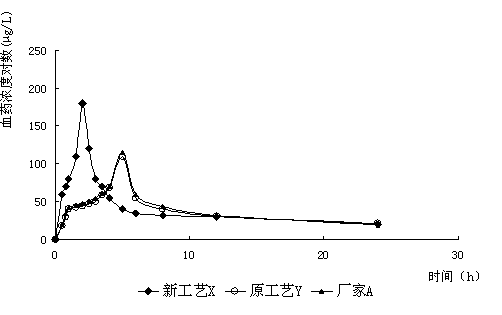
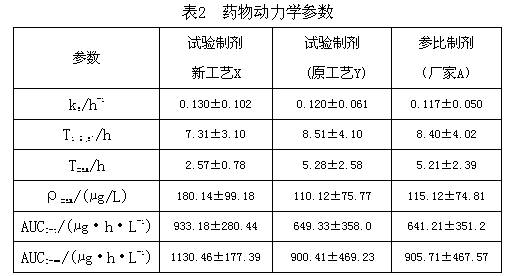
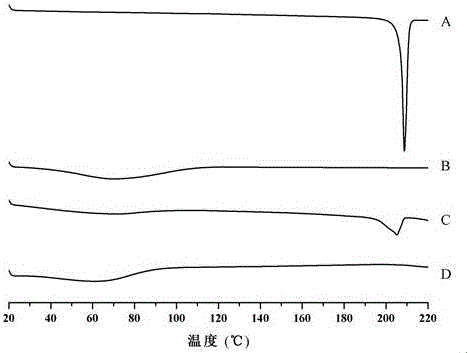
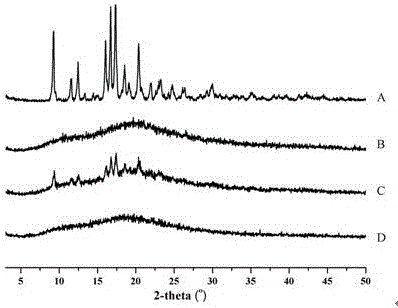
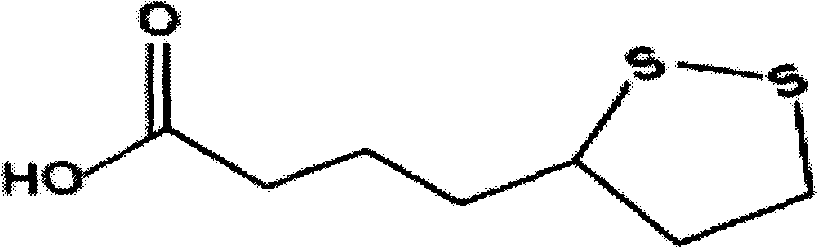
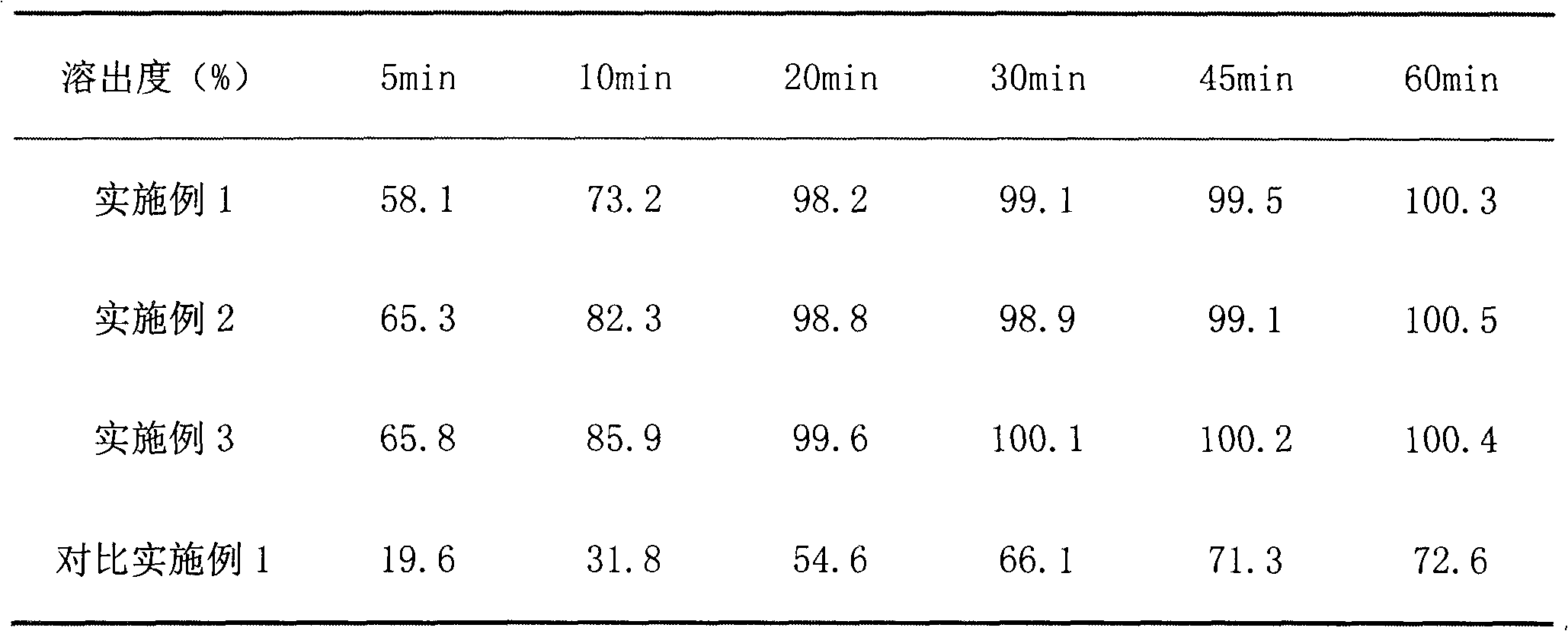
InactiveCN112603900ATreating and Preventing DisordersImprove in vitro dissolutionOrganic active ingredientsCapsule deliveryPolymer scienceAdhesive
The invention discloses a solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid, and the [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]- acetic acid in the solid preparation is a single crystal form with a particle size range of 3-65 [mu] m. The solid preparation also comprises one or more hydrophilic polymer materials as adhesives. The solid preparation disclosed by the invention is high in dissolution rate and bioavailability.
Owner:JIANGSU WANBANG BIOPHARMLS
Terbinafine hydrochloride solid dispersoid and tablet thereof
ActiveCN102641252AImprove in vitro dissolutionReduce dosageOrganic active ingredientsAntimycoticsBULK ACTIVE INGREDIENTPharmaceutical Substances
The invention relates to a terbinafine hydrochloride solid dispersoid, which is composed of terbinafine hydrochloride and carrier materials, wherein the proportion by weight between the terbinafine hydrochloride and the carrier materials is 1 / 2-1 / 15. The invention further relates to a tablet prepared by the terbinafine hydrochloride solid dispersoid. Compared with the prior art, the terbinafine hydrochloride tablet has the advantages of (1) improving dissolution in vitro of terbinafine hydrochloride preparation; (2) solving the problem that the terbinafine hydrochloride is poor in oral administration absorption and low in biological availability; (3) covering harmful odor and irritation of the terbinafine hydrochloride; (4) keeping pesticide effects of large dose while reducing using quantity of active ingredients in the preparation per unit, and reducing drug side-effects; and (5) being simple in preparation process and suitable for industrial production.
Owner:NANJING CHENGONG PHARM CO LTD
Maca flower superfine powder and preparation method thereof
ActiveCN106176872AHigh retention rateSmall granularityPowder deliveryPlant ingredientsFreeze-dryingBULK ACTIVE INGREDIENT
The inventeion discloses maca flower superfine powder and a preparation method thereof. The preparation method comprises the following steps: S1, drying, namely first pre-freezing maca flowers, and performing vacuum freeze drying, so that the moisture content is 3-7%; S2, coarse grinding, namely grinding the dried maca flowers into coarse powder, and standing under the condition of 0-15 DEG C; and S3, superfine grinding, namely introducing air subjected to freeze drying into the coarse powder, and obtaining the maca flower superfine powder by adopting a cell disruption superfine grinding technology, wherein the particle size of the powder is less than 10 microns. According to the invention, the defects that the traditional Chinese medicine health care products are complicated in processing, nutritive materials are lost in the processing process and the like are overcome due to the adoption of a low-temperature disruption superfine grinding method. Pigments, other additives and the like are not added in the operating process, so that the nutrition and flavor substances of the maca flower are remained to the greatest degree, the obtained maca flower superfine powder is pure, natural, safe and sanitary, the retention rate of active ingredients such as macamides, glucosinolate, adenosine, total saponins and the like is high, and the powder is uniform.
Owner:蚌埠启邦科技信息咨询有限公司
Lacidipine-spirolactone co-amorphous solid dispersion and preparation thereof
ActiveCN109010348AImprove in vitro dissolutionImprove stabilityPowder deliveryOrganic active ingredientsSolventSpirolactone
The invention belongs to the technical field of medicines and in particular relates to lacidipine-spirolactone co-amorphous solid dispersion and a preparation method thereof. According to the preparation method, lacidipine and spirolactone, which have a cooperative blood pressure lowering effect, are carriers for each other, and the co-amorphous solid dispersion is prepared by adopting a solvent volatilization method. The co-amorphous solid dispersion is formed by combining the lacidipine and the spirolactone according to the mol ratio of 1 to (1 to 9); Cu-Kalpha radiation is utilized, and a powder X-ray diffraction spectrum shown as a 2theta angle does not have a sharp diffraction peak; peak positions and peak strength of the co-amorphous solid dispersion are obviously changed in a Fourier infrared spectrum. Compared with a lacidipine crystal and a spirolactone crystal, the co-amorphous solid dispersion has the advantages that the dissolution rates and dissolvability of the lacidipineand the spirolactone are remarkably improved. The preparation method of the solid dispersion is simple and feasible and has good repeatability; amplified production is easy to carry out and industrial transformation is carried out; the preparation method has a good clinical application prospect.
Owner:SHENYANG PHARMA UNIVERSITY
Probucol orally administered nanometer solid preparation and preparation method for same
InactiveCN102475688BImprove in vitro dissolutionImprove bioavailabilityPowder deliveryMetabolism disorderHigh dosesDissolution
The invention provides a probucol orally administered nanometer solid preparation, which comprises, by weight, 1 part of probucol, 0.01-0.1 part of additive and 0.05-0.4 part of auxiliary materials. The grain size of the probucol ranges from 10nm to 200nm. The invention further provides a preparation method for the probucol orally administered nanometer solid preparation. The preparation method includes that the probucol and water liquor with 5% of additive are prepared into nanometer suspension with the grain size ranging from 100nm to 600nm by a wet grinding method; the nanometer suspension is homogenized into nanometer grains with the grain sizes ranging from 10nm to 200nm by a high-pressure homogenizing machine; and the nanometer suspension which is homogenized is solidified and is added with the auxiliary materials to be prepared into the orally administered nanometer solid preparation. Compared with a common probucol tablet, the preparation has the advantages that dissolution in vitro of the preparation is increased by 20-30 times, and bioavailability after oral administration is improved by 10-20 times. In addition, the drug loading rate of the preparation is high, and the preparation can meet the requirements of tablet weight clinically needed by high-dose probucol administration. Besides, the preparation is stable, and the dissolution of the preparation can be kept unchanged within two years.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Pharmaceutical composition for edoxaban tablets
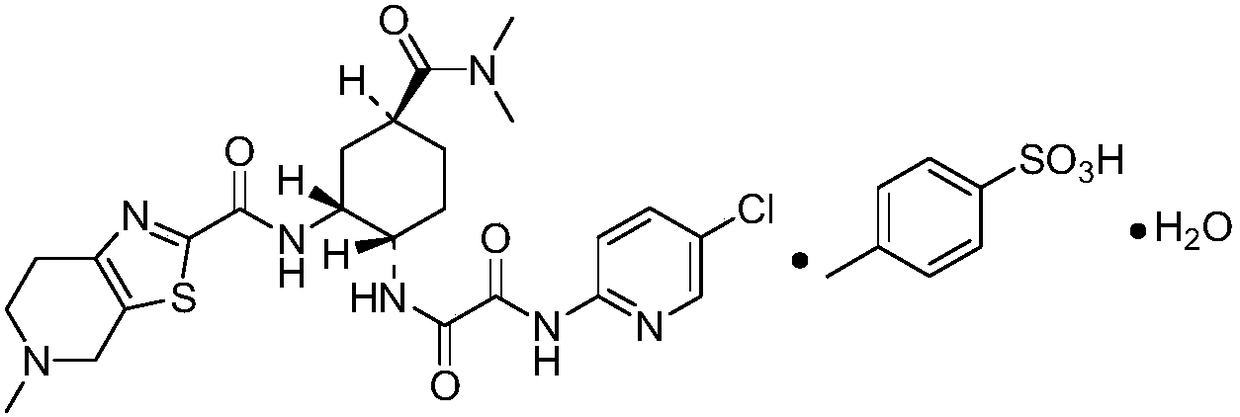
InactiveCN108175753ASignificant progressShort disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineBioavailability
The invention provides pharmaceutical composition for edoxaban tablets. The composition is prepared from 10-50 parts of p-toluenesulfonic acid edoxaban monohydrate, 15-35 parts of a disintegrant, 2-10parts of a 5% polyvinylpyrrolidone 95% ethanol solution, 20-45 parts of filler and 0.5-2 parts of a lubricant, and has the advantages of high disintegration speed, high bioavailability, good stability and the like.
Owner:SICHUAN HAISCO PHARMA CO LTD
Penicillins medicine capsule and preparation method of same
InactiveCN105832694AImprove in vitro dissolutionImprove bioavailabilityPharmaceutical non-active ingredientsCapsule deliveryPenicillin drugBioavailability
The invention provides a penicillin medicine capsule, wherein a capsule shell containing hydroxypropyl starch can maintain the capsule to be stable and prevent the materials in the capsule shell from being wetted and penicillin from being degraded, thereby increasing in-vitro dissolubility and bioavailability of the capsule and further improving quality stability of the capsule.
Owner:HAINAN TANGCHENSHIKE BIOLOGICAL TECH CO LTD
Curcumenol solid dispersion and oral solid preparation thereof
InactiveCN107126417AAchieve oral administrationPromote absorptionAntibacterial agentsOrganic active ingredientsSide effectMedicine
The invention discloses curcumenol solid dispersion and an oral solid preparation thereof. The solid dispersion is composed of curcumenol and a carrier material, wherein a weight ratio of the curcumenol to the carrier material is 1:1 to 1:11. The oral solid preparation can be tablets, capsules or granules and has the advantages of low side effects, high bioavailability, high safety, small toxic and side effects, convenience in application and the like.
Owner:LIAONING UNIVERSITY
Solid dispersion and preoral combination of glibenclamide and preparation method
ActiveCN100341495CInhibition of dissolution rateDoes not change the chemical structureMetabolism disorderSulfonylurea active ingredientsSolubilityDispersed media
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid Solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid](https://images-eureka.patsnap.com/patent_img/c7090b28-6282-4ebc-a775-1da026a41479/BDA0002224250910000071.png)
![Solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid Solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid](https://images-eureka.patsnap.com/patent_img/c7090b28-6282-4ebc-a775-1da026a41479/BDA0002224250910000072.png)
![Solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid Solid preparation containing [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)-amino]-acetic acid](https://images-eureka.patsnap.com/patent_img/c7090b28-6282-4ebc-a775-1da026a41479/BDA0002224250910000081.png)