Pharmaceutical composition for improving in-vitro dissolution and liquidity of spironolactone
A technology of in vitro dissolution and spironolactone, which is used in drug combinations, pharmaceutical formulations, extracellular fluid diseases, etc., can solve the problems of low glass transition temperature, complex preparation process, and limited administration routes, and achieves no organic solvent residues. Simple process and improved in vitro dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Screening of carrier species
[0033] Preparation process: Weigh 1 part of spironolactone and 5 parts of carrier material, wherein the carrier materials are copovidone (PVP VA64), polyethylene caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus), polyoxygen Ethylene (PEO), hypromellose (HPMC-E5), hypromellose succinate (HPMCAS), sieve and mix the drug and carrier material evenly to prepare a physical mixture, set the temperature of the extruder After the temperature rises to the set value and stabilizes, set the screw speed to 20 rpm, start the screw, put the above physical mixture into the extruder, melt and extrude to obtain a transparent strip-shaped extruder. out. After the extrudate is cooled, it is pulverized by a high-speed pulverizer and passed through an 80-mesh sieve to obtain pharmaceutical composition granules.
[0034]Weigh an appropriate amount of each pharmaceutical composition granule (equivalent to about 50 mg of ...
Embodiment 2
[0038] The screening of embodiment 2 Soluplus dosage
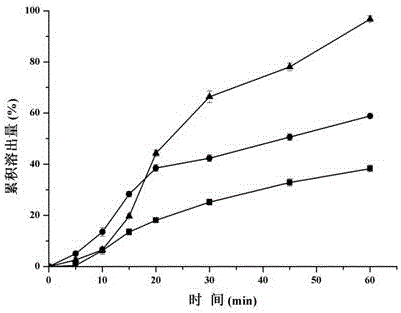
[0039] Set the mass ratio of spironolactone and Soluplus to 1:1, 1:3, 1:5, 1:7, 1:10 respectively, weigh spironolactone and Soluplus according to the prescription ratio, sieve and mix evenly, and prepare a physical mixture, set The extrusion temperature of the extruder is 150°C. After the temperature rises to the set value and stabilizes, set the screw speed to 20 rpm, start the screw, put the above physical mixture into the extruder, melt, extrude A transparent ribbon-like extrudate was obtained. After the extrudate is cooled, it is pulverized by a high-speed pulverizer and passed through an 80-mesh sieve to obtain pharmaceutical composition granules. The dissolution of the pharmaceutical composition particles in water was investigated respectively, and the experimental results are shown in Table 2.
[0040] Table 2
[0041]
[0042]
[0043] It can be seen from the experimental results that when the mass ratio o...
Embodiment 3
[0044] Embodiment 3 extrusion temperature screening
[0045] Weigh 1 part of spironolactone and 5 parts of Soluplus, sieve and mix evenly to prepare a physical mixture, set the extrusion temperature at 130°C, 140°C, and 150°C respectively, after the temperature rises to the set value and stabilizes, set the screw The rotation speed is 20 rpm, the screw is started, and then the physical mixture is put into the extruder, melted and extruded to obtain a transparent strip-shaped extrudate. After the extrudate is cooled, it is pulverized by a high-speed pulverizer and passed through an 80-mesh sieve to obtain pharmaceutical composition granules. The dissolution of the pharmaceutical composition in water was investigated respectively, and the experimental results are shown in Table 3.
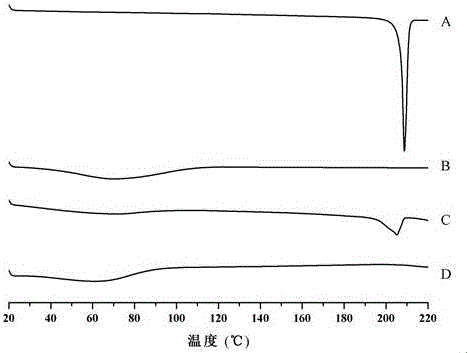
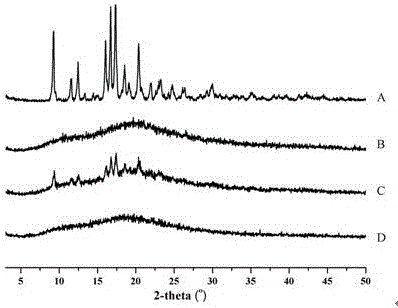
[0046] Investigate the related substances of spironolactone in the pharmaceutical composition respectively. Weigh an appropriate amount of extruded particles (equivalent to about 62.5mg of spironol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com